| Child Kidney Dis > Volume 25(1); 2021 > Article |
|
Abstract
Idiopathic renal hypouricemia (iRHUC) is a rare hereditary disease caused by a defect in urate handling of renal tubules. Type 1 renal hypouricemia (RHUC1) is diagnosed with confirmation of a mutation in SLC22A12 gene which encodes a renal urate-anion exchanger (URAT1). The majority of iRHUC patients are asymptomatic, especially during childhood, and thus many cases go undiagnosed or they are diagnosed late in older age with complications of hematuria, renal stones, or acute kidney injury (AKI). We report a case of a 7-year-old boy with subtle symptoms such as general weakness and dizziness and revealed hypouricemia and incidental nephrolithiasis. Homozygous mutations were detected in the SLC22A12(c.774G>A) by molecular analysis. The present case suggests that fractional excretion of uric acid (FEUA) screening could be better followed by the coincidental discovery of hypouricemia, to prevent conflicting complications of iRHUC, even with normal urine uric acid to creatinine ratio (UUA/UCr), and sequential genetic analysis if needed.
Idiopathic renal hypouricemia (iRHUC) is characterized by impaired uric acid transport, reabsorption insufficiency, or accelerated secretion of uric acid [1,2]. There are two types of iRHUC according to two different genetic mutations. A Mutation of SLC22A12 gene encoding a renal urate-anion exchanger (URAT1) causes iRHUC type 1 while a mutation of SLC2A9 gene encoding a glucose and urate transporter (GLUT9) leads to iRHUC type 2 [3]. Since symptoms of iRHUC are mostly mild or nonspecific, diagnosis is usually made after complications have developed [4]. Occasionally hematuria, nephrolithiasis, or exercise- or dehydration-induced acute kidney injury (AKI) could be initial symptoms [5,6]. Symptomatic iRHUCs are known to be relatively more frequent in homozygous SLC2A9 mutations than SLC22A12 mutations. We present a case of a 7-year-old boy who was diagnosed iRHUC with SLC22A12 mutations by hypouricemia and unexpected renal stone.
A 7-year-old boy visited the outpatient clinic due to persisting dizziness and general weakness from a few years ago. He was born out of normal vaginal delivery and his past medical history since birth was nonspecific except for an event of gross hematuria when he was 7-month-old. But urinalysis at the time showed no hematuria and the symptom never recurred. At his first visit, height was 132 cm (75th percentile), weight 26 kg (25ŌĆō50th percentile), and BMI 14.9 kg/m2 (10ŌĆō25th percentile). His vital signs were normal. However, dizziness and general weakness had been aggravated nearly to syncope that the patient was frequently absent from school. Otherwise, there were no specific symptoms and physical examination also showed no abnormal findings including costovertebral angle tenderness. Laboratory findings were Hb of 11.9 g/dL (normal range; 11.0ŌĆō16.0 g/dL), blood urea nitrogen of 14.7 mg/dL (5.0ŌĆō18.0 mg/dL), creatinine of 0.53 mg/dL (0.22ŌĆō0.59 mg/ dL), and serum sodium of 137 mmol/L (134ŌĆō143 mmol/L). The following parameters were within normal limits: renin, aldosterone, and thyroid function test. However, serum uric acid level was 0.85 mg/dL (normal range; 2.0ŌĆō5.0 mg/ dL), which is abnormally low. Repeated test of uric acid consistently showed low level (0.62 mg/dL). A sequential spot urine test revealed urine uric acid to creatinine ratio (UUA/UCr) of 0.58 but fractional excretion of uric acid (FEUA) of 48.6% (normal range; <10%). Microscopic hematuria was not found on urinalysis. Kidney sonography showed parapelvic high echogenicity and a 4 mm-sized calyceal stone in the left kidney without hydronephrosis (Fig. 1). To identify the underlying cause of the hypouricemia, diagnostic exome sequencing (DES) was performed by Green Cross Genome Corp. using Celemics G-Mendeliome DES kit. Genomic DHA sequencing of the entire coding region of the SLC22A12 was conducted using polymerase chain reaction and the amino acid 258 (tryptophan, TGG) was replaced with a stop codon (TGA) by mutation of G774A [W258X]. The patient was diagnosed a homozygous mutation in the SLC22A12 gene, which is known to be a pathogenic variant of iRHUC (Fig. 2). Accordingly, a whole family including the patientŌĆÖs mother, father, and brother completed DES. The results revealed that his mother and father had heterozygous mutations of the SLC22A12 gene, the amino acid 258 (TGG) replaced with TGA, while his brother had no mutation (Fig. 3). The patientŌĆÖs mother was the only one who had a clinical symptom nonspecific as fatigue. Laboratory findings of her showed low level of serum uric acid, 2.2 mg/dL (normal range 2.6ŌĆō7.2 mg/dL), and high level of FEUA (18.6%). Serum uric acid levels of the patientŌĆÖs father and brother were 4.35 and 4.25 mg/dL, respectively. FEUA of them were 20.4% and 10.4%, each (Table 1). The patient was advised to avoid intense physical activity and dehydration. He has been on conservative management with potassium citrate for a month now and symptoms are similar so far. Also, he is on yearly follow-up, checking serum and urine uric acid level, and kidney sonography.
Hypouricemia is caused by diminished uric acid production or increased uric acid clearance [7]. iRHUC, also known as familial hypouricemia, is an autosomal recessive disorder characterized by serum uric acid level lower than 2 mg/dL due to increased renal clearance with FEUA above 10% [14]. Genetic analysis of the mutations in SLC22A12 and SLC2A9 is a confirmative test. The impact of SLC2A9 mutations on renal excretion of uric acid is reported exceeding that of SLC22A12 [17]. Therefore, patients with mutations in SLC22A12 tend to show relatively minor symptoms, while those with SLC2A9 mutations represent severe symptoms such as hematuria, nephrolithiasis, or AKI. URAT1, encoded by SLC22A12 , is located on the apical surface of the renal proximal tubule and it regulates urate homeostasis. Mutations on SLC22A12 gene decrease the function of URAT1, which leads to renal hypouricemia type 1 [8-10]. Most of iRHUC cases are caused by the mutation in the SLC22A12 . About half are known to be homozygotes while the rest are compound heterozygotes and heterozygotes in order [14]. Of all reported mutations in SLC22A12 , W258X mutation is a predominant genetic cause of iRHUC in Koreans and Japanese [14].
In Korea, several cases of iRHUC caused by SLC22A12 mutations, showing homozygous W258X, are reported (Table 2) [19]. Only one case, Cheong et al. [14], out of four had no symptoms. One patient showing uricosuria was diagnosed at a young age, and the rest cases were detected late after complications developed. Especially, Kim et al. [19] presented a case of familial renal hypouricemia with repetitive exercised-induced AKI. Repetitive complications including AKI might have been prevented if the first diagnosis of renal hypouricemia was made at a younger age and so that patients were given precautions. Unlike existing reports, in the current case, the patient was young, which could be referred to as a lower chance of exposure to intense physical activity, and without typical symptoms associated with complications. However, nonspecific symptoms such as severe general weakness and the past event of red urine which is now presumed to be uricosuria might have been related to the iRHUC. Even random urine test showed uric acid to creatinine ratio (UUA/UCr) within normal range, FEUA turned out to be prominently high and the SLC22A12 mutation was detected. Therefore, patients with an accidental finding of hypouricemia, regardless of UUA/UCr value, should be cautiously evaluated using FEUA. Molecular analysis should also be considered sequentially if needed. In addition, as incidental renal stone coexisted in the patient, unexplained renal stones need close inspections since nephrolithiasis might occur in 10% of patients with SLC22A12 mutations and in 40% of SLC2A9 mutations [13]. Since lab tests and genetic evaluations of the patientŌĆÖs grandparents were not performed, pedigree analysis was not able to be done further. Additional evaluation of his family members could have made the trait and heritability of the disease more clear.
Low level of uric acid, an effective antioxidant acting as a vasodilator, and anaerobic exercises triggering vasoconstriction have a negative synergistic effect on glomerular filtration rate [11]. Thus, sudden onset of AKI can occur a few hours or days after strenuous physical exercises and within the same context, episodes of acute gastroenteritis may cause AKI in patients with renal hypouricemia. Accordingly, in Japan, it is asserted that screening for iRHUC detection using serum uric acid should be done before strenuous physical education classes [12].
There has been a lack of attention for hypouricemia so far because it rarely shows symptoms or requires immediate treatment. Among SLC22A12 mutations, the predominant cause of iRHUC, only few are confirmed with functional significance [18]. However, the W258X mutation, which is relatively common in Korea, tends to express symptoms compared to other subtypes of SLC22A12 mutations. Therefore, most patients with iRHUC are at potential risks for AKI and nephrolithiasis. As the serum uric acid level shows the lowest level between 2.2 and 2.5 mg/dL at 2ŌĆō3 months of age and increases with age [20], children with uric acid level under 2.0 mg/dL should all be excluded from iRHUC.
In short, the current case reports a 7-year-old boy with nonspecific symptoms diagnosed iRHUC as early as before exposure to strenuous physical activities. Also, this report suggests that FEUA, with molecular analysis according to needs, should be done to all patients with hypouricemia to prevent delayed diagnosis and repetitive AKI which might occur.
Notes
Patient consent
This study was approved by the Institutional Review Board of Konyang University Hospital (IRB number 202103-033). Consent was obtained from the patient and his parents.
References
1. Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002;417:447-52.


2. Stiburkova B, Sebesta I, Ichida K, Nakamura M, Hulkova H, Krylov V, et el. Novel allelic variants and evidence for a prevalent mutation in URAT1 causing renal hypouricemia: Biochemical, genetics and functional analysis. Eur J Hum Genet 2013;21:1067-73.



3. Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437-42.


4. Nishizaki N, Fujinaga S, Hirano D, Kanai H, Kaya H, Ohtomo Y, et al. Hereditary renal hypouricemia: a cause of calcium oxalate urolithiasis in a young female. Clin Nephrol 2012;77:161-3.


5. Kamei K, Ogura M, Ishimori S, Kaito H, Iijima K, Ito S, et al. Acute kidney injury after acute gastroenteritis in an infant with hereditary hypouricemia. Eur J Pediatr 2014;173:247-9.


6. Sebesta I, Stiburkova B, Bartl J, Ichida K, Hosoyamada M, Taylor J, et al. Diagnostic tests for primary renal hypouricemia. Nucleosides Nucleotides Nucleic Acids 2011;30:1112-6.


7. Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, et al. Clinical and Molecular Analysis of Patients with Renal Hypouricemia in Japan-Influence of URAT1 Gene on Urinary Urate Excretion. Am Soc Nephrol 2004;15:164-73.

8. Graessler J, Graessler A, Unger S, Kopprasch S. Association of the Human Urate Transporter 1 With Reduced Renal Uric Acid Excretion and Hyperuricemia in a German Caucasian Population. American College of Rheumatology An 2006;54:1.

9. Atsushi K, Keiko I, Hideki W, Ken-ichi S. Akihiko Y. Analysis of Mutations in the Urate Transporter 1 (URAT1) Gene of Japanese Patients with Hypouricemia in Northern Japan and Review of the Literature. Renal Failure 2006;28:3.
10. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radicalcaused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981;78:6858-62.



11. Shimizu Y, Wakabayashi K, Totsuka A, Hayashi Y, Nitta S, Hara K, et al. Exercise-Induced Acute Kidney Injury in a Police Officer with Hereditary Renal Hypouricemia. Case Rep Nephrol Dial 2019;9:92-101.



12. Nakamura A, Niimi R, Yanagawa Y. Renal hypouricemia in schoolaged children: screening of serum uric acid level before physical training. Pediatr Nephrol 2006;21:1898-900.


13. Peris Vidal A, Marin Serra J, Lucas S├Īez E, Ferrando Monle├│n S, Claverie-Martin F, Perdomo Ram├Łrez A, et al. Hipouricemia renal hereditaria tipo 1 y 2 en tres ni├▒os espa├▒oles. Revisi├│n de casos pedi├Ītricos publicados. Nefrologia 2019;39:355-61.


14. Cheong HI, Kang JH, Lee JH, Ha IS, Kim S, Komoda F, et al. Mutational analysis of idiopathic renal hypouricemia in Korea. Pediatr Nephrol 2005;20:886-90.


15. Kim YH, Cho JT. A case of exercise-induced acute renal failure with G774A mutation in SCL22A12 causing renal hypouricemia. J Korean Med Sci 2011;26:1238-40.



16. Han MH, Park SU, Kim DS, Shim JW, Shim JY, et al. A case of idiopathic renal hypouricemia. Korean J Pediatr 2007;50:489-92.

17. Dinour D, Gray NK, Campbell S, Shu X, Sawyer L, Richardson W, Rechavi G, et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol 2010;21:64-72.



18. Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H. A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int 2004;66:935-44.


Fig.┬Ā1.
The kidney sonography of patient shows a renal stone and high echogenicity. A, Calyceal stone* sized (4 mm in size) without hydronephrosis; B, increased echogenicity in parapelvic region.
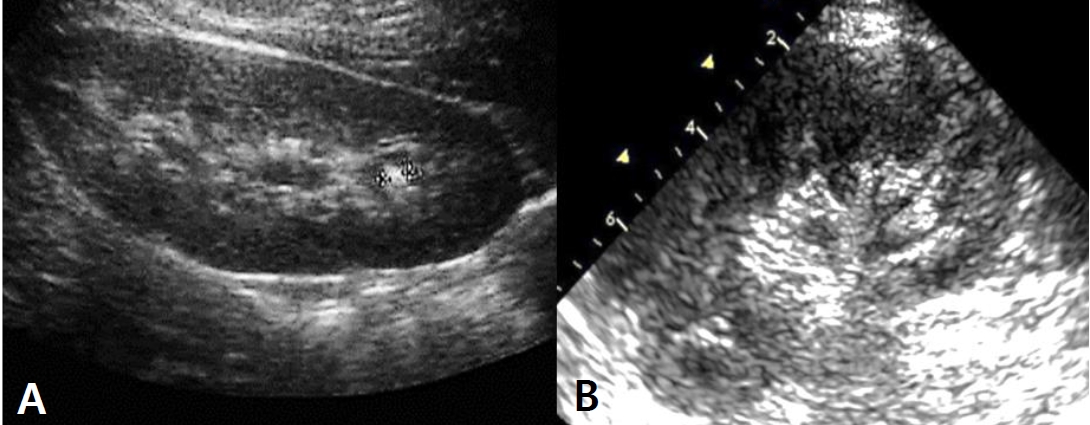
Fig.┬Ā2.
Sequences around the c.774G>A (p.W258X) mutation of the patient and his parents. The black points indicates 774G>A.
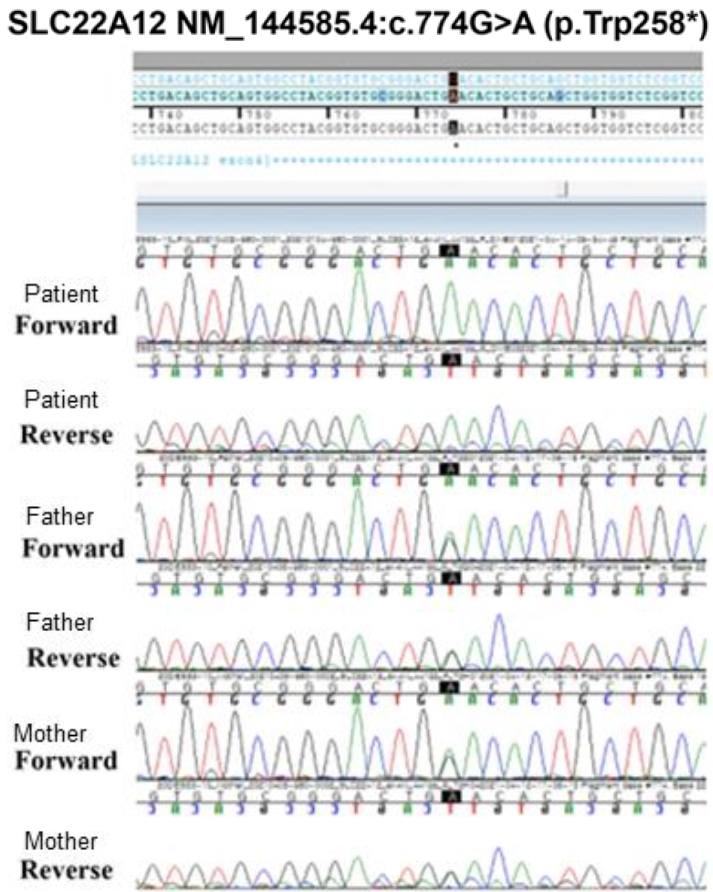
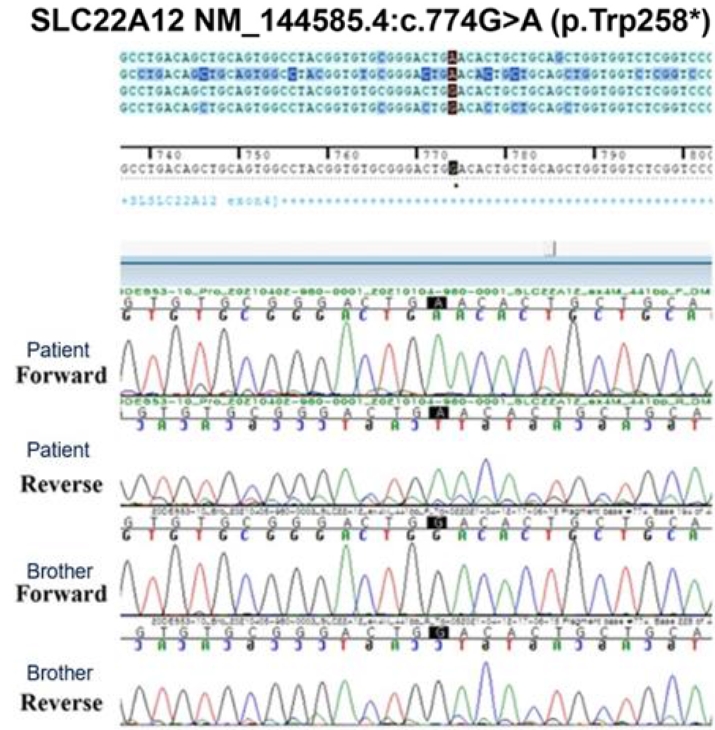
Fig.┬Ā3.
Sequences around the c.774G>A (p.W258X) mutation of the patient and his brother. His brother had no mutation. The black points indicates 774G>A.
Table┬Ā1.
The laboratory findings of the patients and his family
Table┬Ā2.
Reported cases of idiopathic renal hypouricemia with SCL22A12 mutations, homozygous W258X, in Korea
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 4,317 View
- 46 Download
- ORCID iDs
-
Jin Woon Joung

https://orcid.org/0000-0002-4265-9079Young Wha Song

https://orcid.org/0000-0002-6837-0599Jong Dae Kim

https://orcid.org/0000-0002-2606-3646Eun Jung Cheon

https://orcid.org/0000-0003-3844-0864 - Related articles




