Introduction
Rhabdomyolysis means the dissolution of striped (skeletal) muscle, which is characterized by the leakage of muscle cell contents, such as electrolytes, myoglobin, and other sarcoplasmic proteins (e.g., creatine kinase (CK), aldolase, lactate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase) into the circulation [1]. The clinical presentation of rhabdomyolysis varies from an asymptomatic increase in CK to severe acute renal failure and hypovolemic shock [2]. Rhabdomyolysis is commonly caused by crush injuries, medications, seizures and electrolyte disorders [3].
Virus-associated rhabdomyolysis is very rare in adults [3]. However, rhabdomyolysis can also occur after a viral infection especially in children. A few viruses have been reported to be associated with rhabdomyolysis: influenza A/B [2-5], parainfluenza [6-8], coxsackie [9], Epstein-Barr [10], herpes simplex [11], adenovirus [12], and cytomegalovirus [13]. We have experienced the clusters of patients with rhabdomyolysis due to those several viral etiologies during their epidemic periods. Here, we report fifteen pediatric cases of virus-associated rhabdomyolysis.
Materials and methods
A retrospective chart review was conducted based on medical records filed between January 2010 and December 2016 at Asan medical center childrenŌĆÖs hospital. Patients were included in the study if they were aged below 18 years, and had a diagnosis of rhabdomyolysis. Patients who had an underlying disease or previous history of exercise, trauma, drug intoxication or recurrent rhabdomyolysis were excluded. Diagnostic criteria of rhabdomyolysis were based on the increased level of serum myoglobin above the normal range. Patients who had serum creatine kinase (CK) level was above 1,500 IU/L, which is six times the upper limit of normal, were included in this study. Acute kidney injury was diagnosed when estimated creatinine clearance is under 35 mL/min/1.73m 214). All children had increased level of other muscle contents including serum lactate dehydrogenase (LD), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). A multiplex reverse transcriptase polymerase chain reaction for respiratory viruses from a nasopharyngeal aspiration sample was also performed in all patients with the history of viral illness such as fever, cough, rhinorrhea and myalgia, and only those with positive results were finally included in this study.
Whole-body planar bone scintigraphy was performed 4 hours after injection of 1,110├Ś(Body weight in kilograms/70 kg) MBq 99mTc-dicarboxypropane diphosphonate (DPD) in 3 patients to evaluate the severity and to detect the sites of the rhabdomyolysis. Anterior and posterior whole body scans were obtained, respectively using a dual-head gamma camera (Bodyscan; Siemens, Hoffman Estates, IL, or BiadXLT; Trionix, Twinsburg, OH) equipped with high resolution or ultra-high-resolution collimators.
Electrospray tandem mass aminoacid and acylcarnitine analyses was performed in 4 patients for a differential diagnosis of very rare cases of metabolic myopathies [15].
All data were analyzed using the SPSS statistical software version 21 (SPSS Inc., Chicago, IL, USA). Wilcoxon signedrank test was performed to compare the results of laboratory test between onset and the final follow-up.
Results
Fifteen patients were admitted due to rhabdomyolysis between 2010 and 2016. Chief complaints on admission were suddenly developed severe both calf pain causing progressive difficulty in walking (9 patients, 60.0%) and leg weakness (5 patient, 33.3%). The median age was 5.0 years (range 3-9 years). Male to female ratio was 11 to 4. All patients had a history of a recent viral infection from 3 to 10 days (median 5 days) prior to the presentation of rhabdomyolysis. The month of onsets of viral infections were shown in Fig. 1. Clinical symptoms of the infections were mostly fever (15 patients, 100%) with symptoms of upper respiratory infection (13 patients, 86.7%).
All patients received all their routine immunizations. No patient showed red to brown-colored or dark urine. Their causal viruses were influenza virus B (10 patients, 66.7%), influenza virus A (2 patinets, 13.3%), boca virus+ rhinovirus, parainfluenza virus type 1+enterovirus or respiratory syncytial virus (1 patient each, 6.7%).
Their median values and range of laboratory findings were shown in Table 1. Urine myglobin was checked, but only two of them (13.3%) had urine myglobin level above 100 mcg/L. Initial GFR was 157.4 (20.8-275.0) ml/min/1.73 m2. 99mTc-DPD bone scintigraphy was done in 5 patients which revealed increased soft tissue radiotracer uptake in both lower legs and one or both thighs in all of them.
Only one patient (6.7%) had acute kidney injury due to rhabdomyolysis. As initial symptom of rhabdomyolysis, drowsy mentality was presented in this patient. Initial GFR was decreased as 20.8 ml/min/1.73m2. He was treated with dialysis and recovered to normal GFR (138.1 ml/min/1.73 m2) without complication. Influenza A was detected in this patients. Other patients were treated with saline hydration and alkalinization without dialysis. The median hospital days were 3 days (1-10 days) and all patients were discharged within 65 days except 1 patient who had prolonged admission due to acute kidney injury and prolonged increased CK level. All patients were recovered without any complications.
Discussion
The most common cause of rhabdomyolysis was influenza type B in this study. Most reports of influenza associated rhabdomyolysis are about type A [3-5,16]. There are some reports about benign acute childhood myositis associated with influenza B [17], but reports of influenza B-associated rhabdomyolysis are very rare [18-20]. There was a recent report by Wu et al [2] about Taiwanese children with rhabdomyolysis due to influenza B infection. All 24 patients complained of calf pain and recovered without complication during the study periods of 8 years [2], which are similar to our cases.
We had two patients with rhabdomyolysis due to parainfluenza type 1 infection. There are only 4 case reports of rhabdomyolysis associated with parainfluenza virus on a medical online library search in Pubmed (http://www.ncbi. nlm.nih.gov/) so far [6-8,21]. All patients were children, three boys and one girl with ages between 4 and 10 years old. Three of them had parainfluenza type 1 [6,7,21] and the other one had parainfluenza type 3 infection [8]. Three patients developed acute renal failure, and one of them died due to respiratory failure [8]. Parainfluenza virus induced rhabdomyolysis might be more common than expected according to the number of case reports. It usually seems to have a benign clinical course based on our clinical experiences, but sometimes progresses to fatal outcome depending on the circumstances. This discordance of presentation might result from underdiagnosis. If patients have benign clinical course, PCR of CK may not be performed.
We had one patient with rhabdomyolysis associated with bocavirus and rhinovirus coinfection. There was only one report of rhabdomyolysis caused by rhinovirus [22] and there was no report of rhabdomyolysis related to bocavirus so far. This is the first report of the patient with rhabdomyolysis caused by bocavirus.
No patient in this study showed red to brown-colored urine. All the patients had increased serum myoglobin level, but only 2 of them (13.3%) had clinically significant myoglobinuria. Only those with serum myoglobin level above 2,000 ng/mL had myoglobinuria and positive urine occult blood without microscopic hematuria. The classic triad of rhabdomyolysis includes myalgia, red-to-brown or dark urine and muscle weakness [16]. However, only less than 10% of patients with rhabdomyolysis show all three classic features and only 3.6% have dark urine implicating a potentially insidious onset [1,23]. The test for myoglobin in urine might be negative because myoglobin has a short half-life (2-3 hours) and could be rapidly and unpredictably eliminated by hepatic metabolism and renal excretion [24]. So even in patients without red-to-brown or dark urine, we should suspect the possibility of rhabdomyolysis as in our cases.
There was a male predominance in virus associated rhabdomyolysis in this study. All patients were between 3- 9 years old. Patients who had a previous history of exercise or trauma were excluded. In general, it was reported that male patient is usually more prevalent in rhabdomyolysis even in non-exertional, non-traumatic cases such as virus associated rhabdomyolysis [2,25]. This study shows that male sex hormone or any kinds of overactivities or masculine activities which might be expected in boys seem to be related to virus associated rhabdomyolysis.
99mTc-DPD bone scintigraphy revealed increased soft tissue radiotracer uptake in both legs in all five patients who had the test in this study. Phosphate derivative tracers such as 99mTc-DPD, used for bone imaging, are taken up by some soft tissue lesions [26]. Proposed mechanisms for the tracer uptake by these soft tissue lesions are increased calcium concentration and accumulation through the defects in cell membrane after tissue injury, and the tracer binding to hydroxyapatite and calcium phosphate crystals in these tissues [26]. There were relatively clear differences in the amount of uptake and extent of lesions between the patients with higher CK and myoglobin levels and those with lower levels, whereas the scintigraphic findings looked alike between those groups with similar levels regardless of their difference in ages. It seems that 99mTc-DPD bone scintigraphy is very useful in clinically suspected rhabdomyolysis as a sensitive and reliable diagnostic test which enables to localize and quantify muscular involvement.
We did not perform muscle biopsy nor cytokine analysis for the pathogenic evaluations because clinical courses in all patients in this study were benign and recovered without complication. The mechanism of rhabdomyolysis due to viral infection has not been established with certainty yet. The following possibilities have been proposed: First, direct viral invasion of muscle tissue is one possibility; however, the identification of virus or viral particles in affected muscles has been difficult to demonstrate consistently [27]. Second, myotoxic cytokines released in response to viral infection might be another possibility [9,28]. Third, immunologic processes induced by the viral infection could result in muscle damage [27]. It appears that the time from viral illness to the onset of myositis varies, from coincidental to a 3-week delay. In this study, the intervals between viral infection and rhabdomyolysis were from 3 to 10 days. This time course variation seems to support the possibility of different pathophysiologic mechanisms.
There are some limitations in this study. First, not all patients conducted Whole-body planar bone scintigraphy since this study is retrospective study. Second, as this study was conducted in the single center, the selection bias may exist. At last, since sample size is not enough to represent all Korean children, the larger study will be required.
In conclusion, we have experienced patients with rhabdomyolysis due to different kinds of respiratory viral infections, mostly influenza B, followed by parainfluenza and rhinovirus during their outbreaks in the spring of 2010. This is the first report of the simultaneous occurrence of rhabdomyolysis due to multiple viral etiologies. We are not sure if these findings are just coincidental findings by chance or if there are any reciprocal actions among different viruses which favor rhabdomyolysis. This is also the first report of rhinovirus associated rhabdomyolysis.



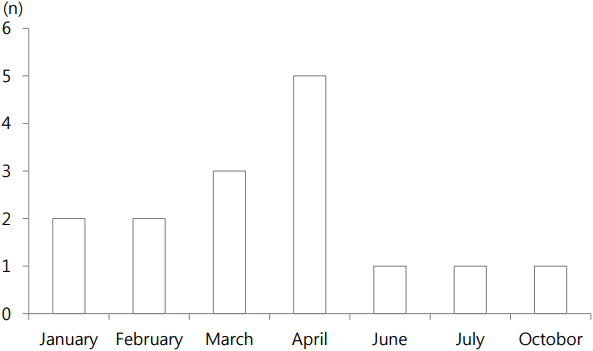
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print