| Child Kidney Dis > Volume 26(2); 2022 > Article |
|
Abstract
Antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis requires prompt diagnosis and treatment, since renal function at the time of diagnosis is significantly associated with renal outcomes. Here, we report two pediatric patients with ANCA-positive glomerulonephritis initially presenting with hematuria, mild proteinuria, and normal renal function. The first patient with a high myeloperoxidase-ANCA titer (>134 IU/mL) was diagnosed with rapidly progressive glomerulonephritis based on renal biopsy and treated with immunosuppressive therapy after 10 months of follow-up. The second patient with a low myeloperoxidase-ANCA titer (11 IU/mL) maintained normal kidney function without medication. Two cases showed different clinical course according to ANCA titer.
Rapidly progressive glomerulonephritis (RPGN) is a clinical syndrome with progressive loss of kidney function that occurs within days to a few months [1], a rare disorder in children. The histopathological diagnosis of RPGN is crescentic glomerulonephritis [1], characterized by epithelial crescents involving 50% or more glomeruli, which clinically manifests as hematuria, variable degrees of proteinuria, oliguria, hypertension, and edema, and glomerular filtration rate (GFR) is decreased at diagnosis in almost all cases. The extensive glomerular crescent formation may lead to kidney failure or death [2]. Prompt and precise diagnosis of antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis is necessary for immediate initiation of appropriate treatment because renal function at the time of biopsy is a strong predictor of renal outcomes [1,3]. Although numerous studies have suggested that serum ANCA titers may aid in the prediction of relapse [2,4,5], no study has reported whether serum ANCA titers may have utility in early diagnosis and determination of treatment. Here, we report two pediatric patients who presented with hematuria, varying degrees of proteinuria, and normal renal function; the disease course was different between the two patients who were ANCA-positive.
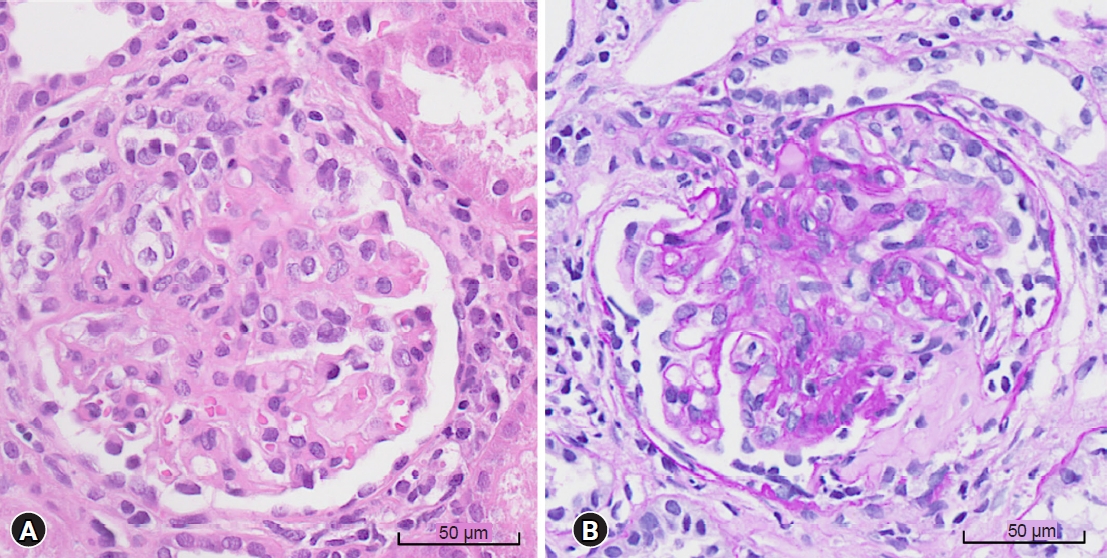
An 11-year-old boy presented with asymptomatic microscopic hematuria and mild proteinuria, which were detected in a school screening program. He had no specific medical or family history of kidney disease. His blood pressure was 107/74 mmHg, the estimated GFR (eGFR) was 103 mL/min/1.73 m2 based on the Schwartz equation in children, and the myeloperoxidase (MPO)-ANCA titer was >134 IU/mL (reference range: <3.5 IU/mL). Other laboratory and ultrasonographic findings were nonspecific. There were 11 to 20 red blood cells per high-power field (RBC/HPF) in urinalysis, and the urine protein-to-creatinine ratio (UPCR) was 0.34. During regular follow-up, proteinuria gradually worsened. Ten months later, he was hospitalized with worsening proteinuria, hematuria, and newly developed rash on both legs during regular follow-up. His blood pressure was 116/71 mmHg, eGFR was 86 mL/min/1.73 m2, and the MPO-ANCA titer was >134 IU/mL. Urinalysis indicated many RBC/HPF, and the UPCR was 1.72. Renal biopsy examination revealed focal extracapillary proliferative glomerulonephritis and necrotizing glomerulonephritis, with cellular crescents in 34 of the 104 glomeruli (Fig. 1). Immunofluorescence and electron microscopy did not show immune deposits. He was treated with methylprednisolone (1,000 mg/day for 3 consecutive days), azathioprine (50 mg/day), and an angiotensin receptor blocker. After 17 days of azathioprine, urinalysis showed increased proteinuria (UPCR, 2.13). Azathioprine was changed to mycophenolate mofetil (500 mg/day), which was maintained over 3 years. One month after using azathioprine, the eGFR and proteinuria normalized, the MPO-ANCA titer decreased to <30 IU/mL, and hematuria disappeared.
A 13-year-old girl presented with asymptomatic microscopic hematuria. She had no specific medical history. Her mother had a history of hematuria due to a renal stone. Her blood pressure was 98/65 mmHg, eGFR was 151 mL/min/1.73 m2, and the MPO-ANCA titer was 11 IU/mL (reference range: <3.5 IU/mL). Urinalysis showed 6 to 10 RBC/HPF, and the UPCR was 0.12. The urine calcium-to-creatinine ratio was 0.045. Other laboratory and ultrasonographic findings were nonspecific. She has been on routine follow-up without medication for 3 years with isolated microscopic hematuria and normal renal function. Her MPO-ANCA titer was not negative but has persistently remained low below 20 IU/mL.
In this study, the MPO-ANCA titer measured during the initial workup differed between the two pediatric patients, both of whom presented with hematuria. The initial evaluation of pediatric patients with asymptomatic hematuria includes medical history, physical examination, urinalysis, blood tests, and renal ultrasonography [6]. Renal biopsy is performed in patients with persistent glomerular hematuria accompanied by significant proteinuria or deteriorating renal function [6]. ANCA-associated glomerulonephritis is rare, and measuring ANCA titers may be optional for the initial workup. In our hospitals, there are two types of ANCA titer test: indirect immunofluorescence, a qualitative test that can confirm perinuclear ANCA and cytoplasmic ANCA, and fluoroenzyme immunoassay, which can confirm anti-proteinase 3 and anti-MPO, a quantitative test. Both patients were tested with fluoroenzyme immunoassay. If a high ANCA titer is measured along with proteinuria, ANCA-associated glomerulonephritis should be considered. Differential diagnosis for positive ANCA includes other immune diseases such as inflammatory bowel disease, rheumatoid arthritis, infection, and connective disease [7]. Therefore, clinical correlation is necessary when interpreting the results. If there is a possibility of ANCA-associated glomerulonephritis, the prognosis of pauci-immune RPGN can be very poor, and early diagnosis and intensive treatment are crucial to prevent progression to end-stage renal disease. A positive ANCA test demands a shorter monitoring interval.
Renal biopsy was performed in the first patient with a high MPO-ANCA titer and aggravating proteinuria but not in the second patient with a low MPO-ANCA titer with asymptomatic microscopic hematuria. The first patient was diagnosed as RPGN associated with ANCA-associated vasculitis referring to the fact that proteinuria worsened and GFR decreased on follow-up. His clinical course seemed to be rather prolonged considering the diagnosis of RPGN. Still, we suppose we could have diagnosed in the very early phase of RPGN before the rapid progression due to the early detection of ANCA on routine surveillance tests. Skin rash on the legs can also be considered as vasculitis. Renal biopsy is the gold standard for RPGN diagnosis [8]. Although the early diagnosis of RPGN is essential to protect against progression to end-stage renal disease, RPGN can be diagnosed only in 30% of pediatric patients through renal biopsy performed early in patients with normal renal function [9]. Early renal biopsy may not be appropriate in ANCA-positive patients with microscopic hematuria and normal renal function. In clinical practice, the diagnosis may be based on clinical manifestation and positive ANCA serology especially in the early phase of RPGN [10].
The clinical course differed between the two patients with different MPO-ANCA titers. The first case with a high MPO-ANCA titer exhibited increasing proteinuria and decreasing renal function several months after the initial presentation, in contrast to the second case with a persistently low MPO-ANCA titer. Several studies have reported that the elevation in ANCA titers is pathogenic and associated with extensive disruption of glomerular capillary walls [2]. The ANCA titer has also been reported usually high at the time of biopsy or during the active disease phase and may disappear several months after treatment [11]. However, not only the ANCA titer but also other ANCA-associated factors, such as the ANCA IgG subclass, the epitope profiles, and the ability of ANCA to bind to its natural inhibitor, may be associated with renal histopathology, disease severity, and clinical course [12]. Nevertheless, patients with higher ANCA titers are more likely to be diagnosed with vasculitis [10]. ANCA is also helpful in monitoring disease relapse, which is common in ANCA-associated glomerulonephritis. In a study of 246 patients with ANCA-associated glomerulonephritis, Booth et al. [4] reported that relapse occurred in 34% of patients after a median of 13 months. A rise and persistently positive ANCA titer are significantly associated with relapse in patients with renal involvement [5]. Recent studies have revealed that the probability of major recurrence is low in patients who became ANCA-negative during follow-up [2].
The first patient was initially treated with methylprednisolone pulse therapy, which was switched to oral steroid therapy, and azathioprine, which was changed to mycophenolate mofetil; this treatment approach resulted in an excellent clinical outcome. Due to its rarity, there are no standard treatment protocols for ANCA-associated glomerulonephritis in children. High-dose intravenous cyclophosphamide and glucocorticoids are conventional treatments commonly used to induce remission in adults [13]. Recent studies suggest that plasma exchange with immunosuppressants in severe cases results in early improvement of renal function by removing circulating ANCAs [14]. Azathioprine, mycophenolate mofetil, or methotrexate s recommended for maintenance therapy in patients at risk of recurrence because of treatment-related toxicities, such as infection, infertility, and malignancy [13]. Cyclosporin is not recommended due to its nephrotoxicity. In case of relapse following cyclophosphamide treatment, rituximab with a reduced risk of nephrotoxicity is recommended [15].
In the first patient with high MPO-ANCA titers, renal biopsy was performed after increased proteinuria was detected and immunosuppressive treatment was initiated. A relevant question is whether treatment should be postponed until the diagnosis of ANCA-associated glomerulonephritis is confirmed through renal biopsy performed following disease progression. Early initiation of immunosuppressants may beneficial for patients with proteinuria and persistently high ANCA titers despite normal renal function and can improve the clinical outcome and reduce drug toxicity. The utility of ANCA titers in predicting relapse and routine measurement of ANCA titers during follow-up remains debatable since an increase in ANCA titers does not always indicate a relapse. The question remains whether initiating immunosuppressant treatment in advance in patients with increasing ANCA titers is beneficial even without other clinical signs, such as proteinuria. In patients at risk for disease relapse, changes in ANCA titers may be an indication for treatment, but future studies are warranted.
In conclusion, ANCA-associated glomerulonephritis progresses rapidly and often relapses, and delayed treatment leads to a poor prognosis. Detection and measurement of serum ANCA titers along with clinical features may facilitate early diagnosis and prognostic prediction in ANCA-associated glomerulonephritis. Although pediatric ANCA-associated glomerulonephritis is rare, persistently high ANCA titers might be related to the progression of kidney disease. Pediatric patients with low ANCA titers may follow a benign clinical course.
Notes
Ethical statements
This study was approved by the Institutional Review Board of Asan Medical Center ChildrenŌĆÖs Hospital (IRB No. 2022-0350), and informed consent was waived due to the retrospective study design.
References
2. Hauer HA, Bajema IM, Van Houwelingen HC, Ferrario F, Noel LH, Waldherr R, et al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int 2002;62:1732-42.


3. Vergunst CE, van Gurp E, Hagen EC, van Houwelingen HC, Hauer HA, Noel LH, et al. An index for renal outcome in ANCA-associated glomerulonephritis. Am J Kidney Dis 2003;41:532-8.


4. Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003;41:776-84.


5. Fussner LA, Hummel AM, Schroeder DR, Silva F, Cartin-Ceba R, Snyder MR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol 2016;68:1700-10.



7. Guchelaar NAD, Waling MM, Adhin AA, van Daele PLA, Schreurs MWJ, Rombach SM. The value of anti-neutrophil cytoplasmic antibodies (ANCA) testing for the diagnosis of ANCA-associated vasculitis, a systematic review and meta-analysis. Autoimmun Rev 2021;20:102716.


8. Gaffo AL. Diagnostic approach to ANCA-associated vasculitides. Rheum Dis Clin North Am 2010;36:491-506.


9. Hagen EC, Daha MR, Hermans J, Andrassy K, Csernok E, Gaskin G, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. Kidney Int 1998;53:743-53.


10. Houben E, Bax WA, van Dam B, Slieker WAT, Verhave G, Frerichs FCP, et al. Diagnosing ANCA-associated vasculitis in ANCA positive patients: a retrospective analysis on the role of clinical symptoms and the ANCA titre. Medicine (Baltimore) 2016;95:e5096.



11. Kerr GS, Fleisher TA, Hallahan CW, Leavitt RY, Fauci AS, Hoffman GS. Limited prognostic value of changes in antineutrophil cytoplasmic antibody titer in patients with Wegener's granulomatosis. Arthritis Rheum 1993;36:365-71.


12. Baskin E, Bakkaloglu A, Besbas N, Hascelik G, Saatci U, Gok F, et al. Ceruloplasmin levels in antineutrophil cytoplasmic antibody-positive patients. Pediatr Nephrol 2002;17:917-9.


13. Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 2017;12:1680-91.



- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,427 View
- 44 Download
- ORCID iDs
-
Ji Hyeon Lim

https://orcid.org/0000-0002-6136-7362Ji Won Jung

https://orcid.org/0000-0001-5358-7966Heoun Jeong Go

https://orcid.org/0000-0003-0412-8709Joo Hoon Lee

https://orcid.org/0000-0001-8010-3605Young Seo Park

https://orcid.org/0000-0001-7653-2036 - Related articles