BK polyomavirus-associated nephropathy
Article information
Abstract
BK polyomavirus (BKPyV) is a ubiquitous virus residing in the kidney tubules and is clinically significant only in immunocompromised patients. In clinical practice, BKPyV is a causative pathogen of BKPyV-associated nephropathy (BKVAN) in kidney allograft recipients or hemorrhagic cystitis of hematopoietic stem cell transplant recipients. Currently, there is no effective treatment for BKVAN; therefore, careful monitoring and prudent modification of immunosuppression are necessary to prevent BKVAN. In this article, the epidemiology, pathophysiology, and current management strategies for BKVAN are reviewed.
Introduction
BK polyomavirus (BKPyV) is a non-enveloped double-stranded DNA virus that was first isolated from a kidney allograft recipient and described in 1971 [1]. More than 90% of the general population is infected with this virus [2]. Primary infection of BKPyV usually occurs subclinically during childhood, and the virus remains in a latent state in the uroepithelium and renal tubular epithelial cells. Upon immunosuppression, BKPyV is reactivated, leading to tubular cell lysis and viruria. One-third to one-half of those who show viruria (>108 copies/mL) develop BKPyV-DNAemia after 2 to 6 weeks along with tubulointerstitial lesions; half of these patients develop BKPyV-associated nephropathy (BKVAN) after another 2 to 6 weeks, especially if plasma BKPyV loads are >10,000 copies/mL.
BKVAN occurs more commonly with more potent immunosuppression, and it is currently one of the most important causes of kidney allograft failure [3-5]. In addition, this virus is associated with ureteral stenosis and hemorrhagic cystitis [6]. Moreover, sporadic cases of pneumonitis, retinitis, colitis, capillary‐leak syndrome, liver disease, meningoencephalitis, encephalitis, hemophagocytic syndrome, and urothelial cancer caused by BKPyV have been described [7]. BKVAN has a poor prognosis, and it has currently no treatment.
Epidemiology
BKVAN usually occurs within the first 2 years after kidney transplantation (KT). Viruria is first noted in 30% to 40% of KT recipients, with decoy cells positive in 20% to 30%, detectable BKPyV-DNA in 10% to 20%, BKVAN in approximately 10%, and graft loss from BKVAN in approximately 5% [8-11]. Interestingly, BKPyV viruria is identified in only 10% of immune-competent hosts; however, its prevalence is 30% to 60% in immunocompromised hosts. In addition, BKPyV-DNA clears within 2 to 12 hours after allograft nephrectomy for BKVAN, implying the presence of replication foci in the kidney allograft. BKPyV-DNAemia is associated with worse outcomes after KT. The 36-month graft survival rate if BKPyV is detected within 6 months post-KT is 79%, compared with 90% in controls [12]. Risk factors for BKVAN include tacrolimus use, potent immunosuppression, acute graft rejection, male gender, old age, younger age for children, delayed graft function, use of cadaveric graft, previous transplantation, human leukocyte antigen mismatches, ABO incompatibility, highly sensitized status, history of hemodialysis (vs. peritoneal dialysis), and a ureteral stent [13]. In other solid organ transplantations, BKPyV-related complications are not common, although cases have been reported following heart and lung transplantations [14,15].
In hematopoietic stem cell transplant (HCT) recipients, hemorrhagic cystitis occurs in up to one-fourth of patients [16,17], 1-month post-HCT [18] and usually lasts more than a month. BKPyV-DNAemia or viruria, which was associated with acute kidney injury, long-term poor kidney function, and mortality, were noted in 18% and 45% of HCT recipients, respectively, in the first 3 months post-HCT [19].
Pathophysiology
The BKPyV dsDNA is enclosed in a viral capsid comprised of an outer layer of VP1 pentamer and an inner layer of VP2 and VP3 proteins [20]. Its genome is composed of circular dsDNA of approximately 5 kb that contains the early viral gene region, which codes the regulatory large and small tumor antigens promoting cell cycle entry/progression and viral replication, the late viral gene region, which codes the viral capsid proteins VP1, VP2, VP3 for entry and assembly of progeny virions, and the non‐coding control region [8]. Once infection occurs, BKPyV hijacks the host cell’s DNA replication machinery for its own reproduction [20]. Therefore, antiviral agents targeting viral DNA replication are ineffective against this virus. After replication, lysis of the host cells along with inflammation and transition to the latent phase follow. Upon immunosuppression, viral replication resumes, causing acute tubular injury, interstitial nephritis, and severe interstitial fibrosis.
Screening of BKVAN
Since there is no effective treatment for BKVAN, screening for BKPyV is the most important strategy to prevent BKVAN. Recently, the American Society of Transplantation Infectious Disease Community of Practice (AST-IDCOP) recommended a monitoring and management strategy for BKVAN (Fig. 1) [7]. Prospective screening of the plasma or urine can identify early viral replication, permitting early intervention and preventing progression to nephropathy or allograft loss. For screening, plasma DNA load is measured monthly for 9 months, and then every 3 months thereafter for 2 years after KT [5,7,21] or when allograft biopsy is performed for surveillance or as indicated and when unexplained allograft dysfunction develops (Fig. 1). BKVAN is suspected when the BKPyV viral load is >10,000 copies/mL with or without serum creatinine level elevation. Histological findings of tubular atrophy, fibrosis, and inflammatory lymphocytic infiltrates need to be differentiated from those of acute cellular rejection. Intranuclear BKPyV inclusion bodies suggest BKVAN, which can be identified with special staining of large T antigen [22].
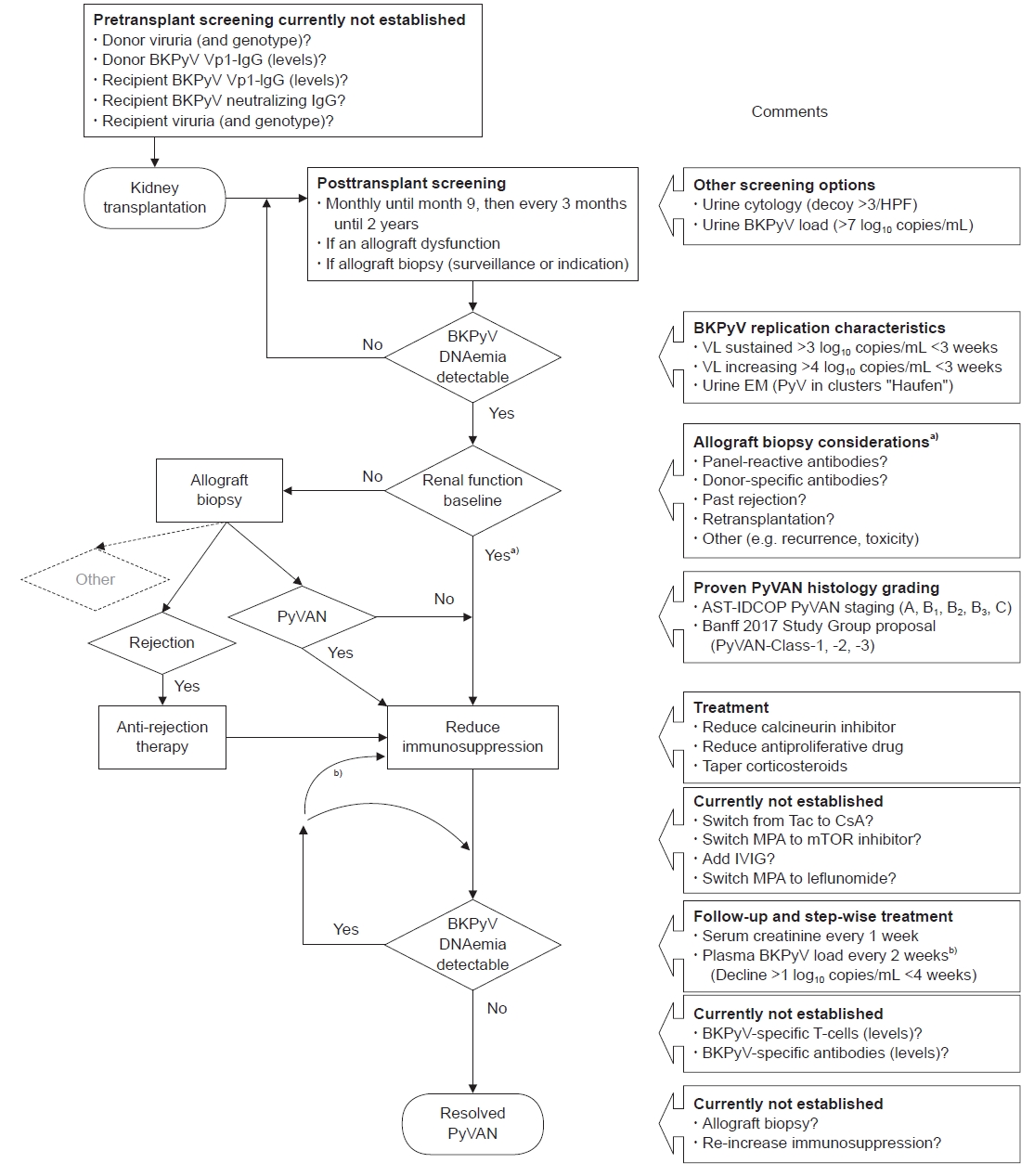
Monitoring and management strategy for BK polyomavirus (BKPyV)-associated nephropathy (BKVAN). HPF, high-power field; VL, viral load; EM, electron microscopy; PyV, polyomavirus; PyVAN, polyomavirus-associate nephropathy; AST-IDCOP, American Society of Transplantation Infectious Diseases Community of Practice; Tac, tacrolimus; CsA, cyclosporine-A; MPA, mycophenolic acid or equivalent; mTOR, mammalian target of rapamycin; IVIG, intravenous immunoglobulin. a)Allograft biopsy should be considered in patients with baseline allograft function, if concurrent (subclinical) acute rejection is likely; b)If decline in plasma BKPyV-DNAemia is <1 log10 copies/mL in <4 weeks, further immunosuppression is required. Reuse from Hirsch et al. Clin Transplant [7] with permission from John Wiley and Sons.
Diagnosis of BKVAN
Diagnosis of BKVAN is confirmed only by allograft kidney biopsy, with features of interstitial nephritis and large T antigen positivity with immunohistochemistry. If the plasma viral load either increases to >10,000 copies/mL in one of two measurements within 3 weeks or is sustained at >1,000 copies/mL in two measurements within 3 weeks, these are considered presumptive or probable BKVAN, respectively, which requires modification of immunosuppression and kidney biopsy if there is a risk of acute rejection and/or impaired kidney function (Fig. 1). Additionally, urine BKPyV viruria >10,000,000 copies/mL or presence of decoy cells indicates possible BKVAN, warranting plasma BKPyV viral load monitoring. If BKVAN is established, immunosuppression needs to be reduced, which can be accomplished even without biopsy confirmation. In 10% to 30% of cases, false-negative results were obtained as biopsies were taken early after BKPyV‐DNAemia onset, and medullary tissue was not sampled [21].
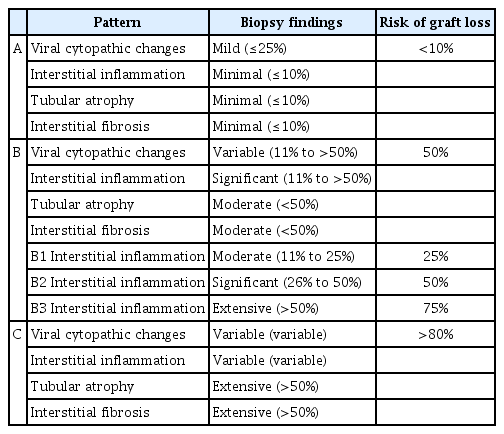
The pathology of BKVAN is described using the histologic patterns of BKVAN proposed by the 2013 AST-IDCOP. In addition to viral cytopathic changes, acute tubular injury, interstitial nephritis, and severe interstitial fibrosis are denoted as patterns A, B, C, respectively, along with the degree of interstitial nephritis (Table 1) [23]. Meanwhile, the Banff 2017 Working Group Classification takes into account the intrarenal PyV load (extent of virally induced tubular changes with intranuclear viral inclusion bodies and/or a positive immunohistochemistry reaction for SV40 T antigen) and Banff ci scores (Table 2) [22]. These histological patterns/classifications indicate the risks of allograft kidney loss, which range from <10% to >80%. In cases wherein there is evidence of rejection or intimal arteritis, or a positive C4d stain is observed, intensifying immunosuppression to treat rejection should first be considered before treating BKAVN.
Treatment
Reduction in immunosuppression
The first-line management for BKVAN is the reduction in immunosuppressive agents (Fig. 1). Usually, a stepwise approach to reduce immunosuppression is adapted; calcineurin inhibitors (CNI) are initially reduced by 25% to 50%, followed by mycophenolate mofetil (MMF) by 50%, and finally MMF discontinuation if there is no improvement [24]. Another approach is initial MMF reduction by 50%, then CNI reduction by 25% to 50%, and finally MMF discontinuation. Steroids are often limited to prednisolone 10 mg or less, and targets of CNI trough levels are <6 ng/mL with tacrolimus and <150 ng/mL with cyclosporine. Additionally, mammalian target of rapamycin (mTOR) inhibitors were shown to decrease BKPyV‐DNAemia and/or BKVAN [25]. Since cyclosporine and sirolimus, an mTOR inhibitor, inhibit BKPyV replication in vitro, switching immunosuppressants from tacrolimus to cyclosporine, CNI to sirolimus, MMF to sirolimus, or MMF to leflunomide can be considered, albeit with weak evidence [8,21]. However, reducing or modifying immunosuppression may be inadequate to prevent rejection, whereas excessive immunosuppression will worsen BKVAN and cause allograft dysfunction, tubulointerstitial nephritis, and fibrosis [7]. Therefore, prior to modifying immunosuppression, patient’s immunological risk, viral load, and kidney dysfunction must be considered [26].
Other management
No randomized clinical study has proven the efficacy of other adjunctive managements aside from modification of immunosuppression.
Intravenous immunoglobulins
Intravenous immunoglobulins, which have indirect immunomodulatory effects, contain high titers of potent BKPyV neutralizing antibodies that can directly neutralize BKPyV activity [7]. For BKVAN, 0.1–2.0 g/kg/dose is used.
Cidofovir
Cidofovir is a nucleoside analog licensed by the U.S. Food and Drug Administration for the treatment of cytomegalovirus retinitis [23]. Its efficacy in BKVAN is controversial; however, cidofovir concentration in renal tissues and urine is high. Therefore, cidofovir can theoretically be effective against viral infection in the kidneys. Coincidentally, drug-induced anterior uveitis has been reported in 12% to 35% of cases. Cidofovir is given as a low-dose regimen at 0.25–1.0 mg/kg/dose every 2 to 4 weeks, and serum creatinine, white blood cell count, ocular and visual symptoms should be monitored every 2 weeks [24].
Leflunomide
Leflunomide is a disease-modifying antirheumatic drug that inhibits dihydroorotate dehydrogenase, which is necessary for pyrimidine synthesis [27]. Its anti-proliferative activity and anti-inflammatory effects are a result of the selective inhibition of mTOR signaling. It has been shown to inhibit BKPyV viral DNA synthesis in vitro [28]. According to a systematic review, clearance of BKPyV‐DNAemia was reported in 33.3% to 92.3% of cases in different studies and 27 (10.1%) graft losses were reported in 267 patients [27,29]. Considering its immune-modulating effects, leflunomide is often used in place of MMF in cases of BKVAN [7]. Adverse events of leflunomide include hepatitis, hemolysis, thrombotic microangiopathy, myelosuppression, and fungal pneumonia. Thus, monitoring the complete blood count and performing liver function tests every 4 weeks is mandated.
Fluoroquinolones
Fluoroquinolones, including ciprofloxacin, inhibit BKPyV replication by affecting the helicase activity of the virus-encoded large T antigen [8]. However, in a randomized controlled trial to determine the effectivity of a 3-month course of ciprofloxacin as BKPyV prophylaxis in KT, ciprofloxacin not only failed to improve the allograft outcome but also increased levels of BKPyV-DNA and incidence of fluoroquinolone-resistant Gram-negative infections [30].
Special consideration for children
Similar to other common infections, children often require immunosuppression before primary infection with BKPyV. Therefore, they are more likely to be BKPyV-seronegative, which increases both the risk, severity, and duration of viral replication [31-34]. Thus, children may benefit more from intravenous immunoglobulins administration [35]. If they are BKPyV-seropositive, this means exposure to BKPyV was a recent event, which is why younger children harbor higher levels of immune effectors. Children with end-stage kidney disease often have urinary tract anomalies, which carry a risk of viral reactivation similar to a ureteric stent [31]. In addition, there may be hyperfiltration due to donor-recipient size mismatch in pediatric KT, which may delay the diagnosis of BKVAN [7]. Therefore, screening in children must be extended to a longer period [7].
Conclusions
BKVAN, although uncommon, threatens allograft survival in KT. Currently, there is no approved and effective treatment for BKVAN. To prevent BKVAN, meticulous screening up to the third year post-KT and appropriate modification of immunosuppression is necessary to improve outcomes.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: YHA, HGK
Project administration: YHA, HGK
Visualization: YHA, HGK
Writing-original draft: YHA, HGK
Writing-review & editing: YHA, HGK
All authors read and approved the final manuscript.