Clinical Characteristics and Long-Term Prognosis of Alport Syndrome: A Retrospective Single-Center Study
Article information
Abstract
Purpose
Alport syndrome (AS) is one of the most common inherited renal diseases caused due to mutations of genes encoding specific proteins of the type IV collagen family, and its major clinical manifestations include progressive renal failure, sensorineural deafness, and ocular abnormalities. We investigated the clinical characteristics and long-term prognosis of AS in Korean pediatric and adult populations.
Methods
We conducted a retrospective review of medical records of 33 children and adults who had been diagnosed or treated with AS from 1985 to 2019.
Results
The mean age of the 33 patients diagnosed with AS was 16.2±13.6 years, and the male-to-female ratio was 2:1. At the first visit, recurrent gross hematuria was the most common initial symptom. In 10 of 33 patients (30.3%), sensorineural hearing loss (SNHL) was diagnosed, but none had ophthalmic problems. Moreover, 11 of 33 patients (33.3%) had advanced to end-stage renal disease (ESRD), and a significant difference was observed in the age of the patients who progressed to ESRD based on the presence or absence of SNHL (P=0.035).
Conclusion
SNHL in AS can be an important prognostic factor for long-term deterioration of renal function. Further investigation is required to confirm the clinical course and the genetic characteristics of AS in Korea through prospective national cohort studies.
Introduction
Alport syndrome (AS) is one of the most common inherited renal diseases caused due to mutations of genes encoding specific proteins of the type IV collagen family [1]. The type IV collagen family is expressed in the basement membrane of the kidney as well as the ear and eye [2]. The major features of AS are progressive renal failure, sensorineural deafness, and ocular abnormalities. Clinically, hematuria is the most common symptom of AS and generally manifests as microscopic or recurrent gross hematuria [1-3]. Proteinuria is absent in the early stages, but it gradually increases with age, and all males with X-linked AS (XLAS) progress to end-stage renal disease (ESRD) [1,2].
AS has three genetic patterns. The majority, comprising approximately 80–85%, show the X-linked dominant form caused due to mutations of the COL4A5 gene. Autosomal recessive or dominant forms caused due to mutation of the COL4A3 or COL4A4 gene have also been reported [4-6]. XLAS can be suspected when a young male has a severe course of illness. Untreated males with XLAS progress to ESRD before the age of 30 years and are frequently accompanied by hearing loss. However, females with XLAS exhibit various disease severities depending on the relative activities of the mutant and the normal X chromosome. Autosomal recessive AS (ARAS) should be suspected in young females who do not have a family history with typical clinical features and severe disease, such as ESRD and deafness [2,7]. The aims of this study were to identify the genetic and clinical characteristics of patients with AS in Korea and to identify the clinical indicators that can predict long-term prognosis
Materials and methods
1. Study design and patients
We retrospectively reviewed the medical records of 33 patients who had been diagnosed or treated with AS at our hospital from 1985 to 2019. AS was diagnosed through investigations such as family history, audiogram, ophthalmic examination, renal biopsy, and genetic study in patients with hematuria or AS family history. Clinical details such as age, sex, familial history, serum creatinine, urinalysis, and estimated glomerular filtration rate (eGFR) were collected. Renal biopsy was performed in 27 of the subjects, and particularly diffuse thickening and multilamellation of the glomerular basement membrane (GBM) on an electron microscope (EM) were used as diagnostic criteria. Genetic testing was performed in 18 of the subjects, and the conventional Sanger sequencing for the COL4A5 gene or next-generation sequencing (NGS) was performed. Hearing abnormality was defined as sensorineural hearing loss (SNHL) of moderate abnormality (41–55 dB) in pure tone audiometry by audiogram. Ophthalmic abnormalities were characterized by anterior lenticonus and maculopathy, which are the characteristic of AS. Renal function was evaluated according to the eGFR value, and ESRD was defined when the eGFR value was less than 15 mL/min/1.73 m2.
2. Statistical analysis
The mean value of each category is reported as mean± SD. The Student’s t-test or the Wilcoxon rank-sum test was used for analyzing continuous variables and nonparametric distributions. The Chi-square test and Fisher’s exact test were used for analyzing categorical variables. The significance level was defined as P<0.05. All statistical analyses were performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, Illinois, USA).
Results
The mean age of the 33 patients who first visited our hospital was 12.7±14.0 years, and the male-to-female ratio was 2:1. Of the 33 patients, 26 (78.8%) were diagnosed before the age of 18 years, with the mean age at diagnosis being 16.2±13.6 years. At the first visit, recurrent gross hematuria was the most common initial symptom (57.6%), followed by proteinuria with persistent microscopic hematuria (PMH) (30.3%) and PMH only (6.1%) (Table 1).
For confirming the diagnosis, 27 (81.8%) patients underwent renal biopsy, genetic tests were conducted in 18 patients (54.5%), and 15 patients (45.5%) underwent both. In the genetic tests, the conventional Sanger sequencing was performed in 14 patients (77.8%) to identify COL4A5 gene mutation, and the remaining 4 patients underwent NGS. Based on the test results, COL4A5 gene mutation was confirmed in 7 patients (38.9%), and 1 patient with COL4A4 gene mutation was confirmed through NGS. In the remaining patients, the COL4A5 gene mutation could not be confirmed by Sanger sequencing or the results of the mutation were unknown.
A total of 18 patients (54.5%) had a family history of kidney disease, such as AS, chronic kidney disease, proteinuria, or microscopic hematuria. On the basis of the results of genetic tests and family history, the specific genetic patterns could be confirmed in only 9 patients, of whom 7 patients had an X-linked pattern, and 2 patients had an autosomal recessive genetic pattern.
Of the 33 patients, 11 (33.3%) progressed to ESRD, with the mean age at ESRD diagnosis being 24.1±10.9 years. The mean duration of progression to ESRD was 14.0±5.8 years from the diagnosis of AS. The 8 patients (72.7%) with ESRD underwent kidney transplantation, and 3 of them are on hemodialysis. None of the patients were diagnosed with ophthalmic problems, but there were 10 patients (30.3%) with SNHL. The mean age at the diagnosis of SNHL was 21.5 years (Table 1 and 2).
We divided all the patients with AS into 26 pediatric patients diagnosed at age <18 years and 7 adult patients diagnosed at age ≥18 years. In pediatric patients, recurrent gross hematuria was the most common cause of the first visit, accounting for 73.1%, whereas proteinuria with PMH was the most common in adults (71.4%). In the diagnostic method, 23 of the 26 children (88.5%) were biopsied and 17 (65.4%) underwent genetic study, whereas in the adult group, 3 of 7 adults (42.9%) were biopsied and only 1 (14.3%) underwent genetic study. However, only 17 of the 23 children who underwent biopsy actually met the diagnostic criteria of AS and were subjected to more genetic tests than adults (Table 3).

Comparison of General Characteristics and Laboratory Features according to the Age of Diagnosis of Alport Syndrome
When compared with the presence or absence of proteinuria as an initial symptom, no significant difference was detected in ESRD progression between the two groups. In contrast, SNHL was found at a significantly higher frequency in the ESRD group (P=0.003, Fisher’s exact test) (Table 4). A significant difference was observed in the age of the patients who progressed to ESRD according to the presence or absence of SNHL (P=0.035) (Fig. 1).
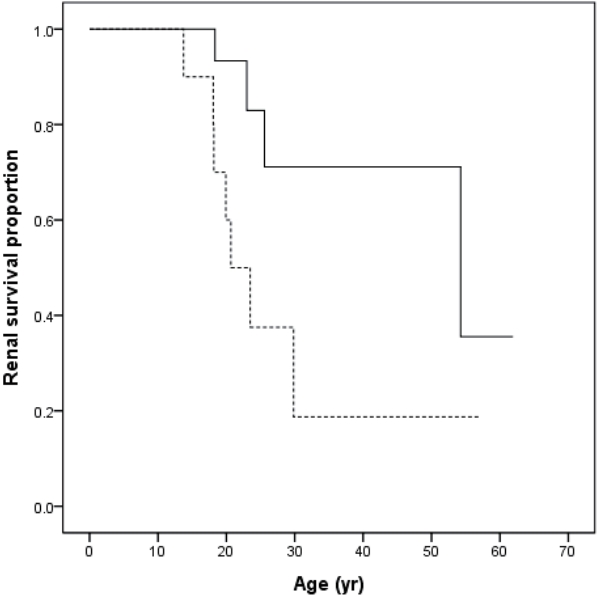
Comparison of the time taken to proceed to ESRD according to the presence or absence of SNHL. Solid line indicates patients without sensorineural hearing loss (n=23). Dots indicate patients with sensorineural hearing loss (n=10) (P=0.035). Abbreviations: ESRD, end-stage renal disease; SNHL, sensorineural hearing loss.
Discussion
AS is a hereditary kidney disease caused due to mutation of a specific protein of the type IV collagen family constituting the basement membrane. The disease is accompanied by progressive renal failure, hearing loss, and ocular abnormalities, and its prevalence has been estimated at 1 in 5,000 people in the United States or Europe. AS accounts for 0.5% of adults and 12.9% of children among all patients with ESRD [7,8]. Most of the clinical symptoms in patients with AS manifest as recurrent gross hematuria and PMH before the age of 5 years. Proteinuria is uncommon at a young age, but it gradually increases with age [5,7,8]. In our study, the male-to-female ratio was 2:1, and 26 patients (78.8%) visited the hospital before the age of 18 years. At the first visit, recurrent gross hematuria was the most common initial symptom (57.6%). When the symptoms were divided into hematuria and proteinuria, hematuria was observed in all patients, whereas proteinuria was found in only 33.3% of the patients. Jais et al. reported that 75.2% of patients with AS in Europe had proteinuria and 95% had hematuria [9,10]. In Japan, Yamamura et al. reported proteinuria in 72.6% and hematuria in 97.9% of patients [11]. Furthermore, Chugh et al. reported proteinuria and hematuria prevalence rates of 31.7% and 96.8%, respectively [12]. Although there was a slight difference, hematuria was the most common in the majority of cases.
AS is diagnosed in patients with hematuria based on EM results of renal biopsy showing a multilamellation of GBM, and family history, SNHL, and ocular abnormalities are helpful for diagnosis [2,3,7]. In our study, family history could be confirmed in 54.5% of the patients, and 27 patients (81.8%) underwent renal biopsy, whereas only 18 patients (24.2%) were genetically tested. Genetic study was performed in 65.4% of pediatric patients, whereas among the adults, only 1 patient (14.3%) underwent genetic test. Moreover, among adults, the diagnosis was based on family history and clinical symptoms rather than renal biopsy or genetic testing. Inheritance could be confirmed in 9 patients based on family history and genetic study results, of whom 7 (77.8%) had XLAS, 2 (22.2%) had ARAS confirmed, whereas ADAS was absent. In an earlier study of patients in China, Wei et al. reported that 89.7% of those with AS had XLAS and 10.3% had ARAS [13].
In particular, the results of renal biopsy at a young age or in females can be ambiguous, which poses a limitation to diagnosis based on biopsy alone; moreover, the diagnosis becomes further complicated due to the difficulty in confirming the exact family history [2,7,14]. Therefore, it is necessary to suspect AS if there is persistent hematuria of unknown etiology at a young age. In our study, only 17 of the 23 pediatric patients who underwent renal biopsy were diagnosed by biopsy, implying that genetic testing was performed in more children than adults. Furthermore, as genetic patterns cannot be discriminated based on only the abnormalities observed in EM findings, genetic study is essential to confirm the exact genetic pattern [2]. Because it is known that the majority of AS is caused due to mutations of the COL4A5 gene, performing conventional Sanger sequencing of COL4A5 is cost-effective [4]; however, NGS also has advantages as the possibility of mutation of the COL4A4 gene cannot be ruled out [15]. Moreover, the results of genetic testing are more important because various phenotypes can be displayed depending on the mutation position or the mutation type. Through NGS, it is easy to confirm large deletions and insertions in female patients, which are difficult to confirm by Sanger sequencing, and will help confirm inheritance in patients in whom it is difficult to confirm the pedigree. In countries with a large number of renal donations among relatives, such as Korea, it is believed that genetic testing for asymptomatic female carriers will help determine whether the donor has AS [4]. In the results of this study, it can be confirmed that the diagnosis of AS in adults is based on clinical symptoms, so that biopsy or genetic testing was rarely performed. Therefore, since genetic testing is important for identifying genetic patterns and predicting prognosis, it is essential to diagnose AS patients.
The prognosis for AS is poor, with 90% of males with XLAS and the majority of those with ARAS progressing to ESRD before age 30 years [7,10,15]. No specific treatments are available till date, and recent studies have demonstrated that inhibition of the renin-angiotensin-aldosterone system using angiotensin-converting enzyme inhibitor or angiotensin receptor blocker slows the progression of the disease by reducing proteinuria. Dialysis or renal transplantation is performed when the patient reaches ESRD [2,7]. Most of the patients with AS who progress to ESRD have a better prognosis than those with other kidney diseases. However, 2–5% of transplant patients are likely to have graft failure due to post-transplant anti-GBM nephritis caused due to alloantibodies to the donor kidney GBM [7,16,17]. The factors associated with the prognosis of AS include gender, family history, age at onset, hearing loss, ocular abnormalities, and abnormalities in EM findings [1,14]. A study that analyzed the risk factors associated with long-term prognosis of AS in Korea reported hypertension, edema, proteinuria, and GFR at the time of diagnosis as the important factors in predicting prognosis [1]. Jais et al. reported that 78.3% of patients with XLAS progressed to ESRD, and 90% of patients progressed to ESRD before age 40 years. In addition, SNHL was reported in 28% of patients with XLAS in Europe, and ophthalmic complications accounted for 15% [9]. On the other hand, Wei et al. reported that 47 of 126 (37.3%) patients progressed to ESRD, 38.9% of those in the XLAS group and 23.1% of those in the ARAS group had renal failure. SNHL was reported in 59.8% of the patients, with males being more significantly affected than females [13,18]. In the present study, 11 patients (33.3%) had ESRD, and the mean age at the time of ESRD diagnosis was 24.1±10.9 years. Moreover, there were 30.3% of patients with SNHL. In a previous study, Chugh et al. reported that there was no relationship between ESRD frequency and hearing loss and ophthalmic complications [12]. However, in our study, SNHL was found to be more frequent in the ESRD group (P=0.003) and there was a significant difference in the age of the patients who progressed to ESRD based on the presence or absence of SNHL (P=0.035). Jais et al. reported that hearing loss and proteinuria were significantly related to ESRD progression [9], whereas no significant relationship was confirmed between proteinuria and ESRD in our study.
The present study has some limitations. Due to its retrospective design and being conducted in a single center, it was difficult to identify the exact inheritance pattern and the type of gene mutation because the diagnostic processes of the enrolled patients were not consistent and most physicians to treat adults with AS tend to not perform the genetic test for AS. However, by analyzing the clinical symptoms of pediatric and adult patients simultaneously, it was possible to analyze the diagnostic process and the long-term prognosis of AS in Korea. Moreover, our study confirmed that SNHL is an important prognostic factor for long-term deterioration of renal function. In addition, in order to confirm the correct inheritance related to the prognosis of AS, it is necessary to actively conduct genetic testing at diagnosis. Therefore, further research is needed to confirm the clinical course and the genetic characteristics of AS in Korea through prospective national cohort studies.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Patient consent
This study was approved by the Institutional Review Board of the Kyungpook National University Hospital (IRB No. 2020-07-054).



