Predictive Markers for Screening Renal Damage in Children with Urinary Tract infections and Vesicoureteral Reflux
Article information
Abstract
Purpose
Urinary tract infections (UTIs) are the most common and serious bacterial infections in children. Therefore, early diagnosis of vesicoureteral reflux (VUR) for treatment planning and the identification of noninvasive markers that can predict renal injury are important in patients with UTIs. We analyzed the clinical features of pediatric UTIs commonly encountered by general practitioners and reinterpreted the blood tests and imaging findings to identify the important clinical predictive markers of VUR in order to selectively perform VCUG.
Methods
This retrospective study was performed among 183 children diagnosed with a UTI or acute pyelonephritis.
Results
The most significant predictor of high grade and bilateral VUR identified using area under the curve analyses was hydronephrosis on kidney ultrasound images with renal cortical defects on dimercaptosuccinic acid (DMSA) kidney scan simultaneously, followed by hydronephrosis only on kidney ultrasound.
Conclusion
The presence of hydronephrosis on kidney ultrasound images or cortical defects or asymmetric kidneys on the DMSA kidney scans can be predictive markers of VUR, reducing the need for VCUG. Our study can thus help minimize the exposure to radiation among patients through selective VCUG.
Introduction
Urinary tract infections (UTI) are the most common and serious bacterial infections in children [1]. Unlike lower UTIs, upper UTIs can cause renal scars and contribute to the development of hypertension and end-stage renal disease if not treated properly [2,3]. UTIs are known to present many risk factors, especially vesicoureteral reflux (VUR), which is a common malformation found in approximately 30% of young females with UTIs [4]. VUR can cause recurrent UTIs as well as damage and scarring of the kidneys, which can lead to reflux nephropathy [5,6]. Therefore, early diagnosis and treatment of VUR is critical for a better outcome [7].
To diagnose a VUR, voiding cystourethrography (VCUG) is performed [8]. VCUG facilitates identification of the precise anatomical structures surrounding the bladder as well as diagnosis of related malformations [9]. However, VCUG is an invasive examination that requires fluoroscopy and involves inserting a Foley catheter and injecting a contrast agent. Invasive procedures performed in infants and children are painful, and may worsen the emotional or mental state and physical exhaustion of the caregivers [10,11]. Therefore, it is important to identify noninvasive markers in patients with UTIs that can predict renal injury without the need for VCUG findings [12]. We analyzed the clinical features of pediatric UTIs commonly encountered by general practitioners and reinterpreted the blood tests and imaging findings to identify the important clinical predictive markers of VUR in order to allow for the selective implementation of VCUG.
Materials and methods
This retrospective study was performed with 183 children under 15 years of age diagnosed with 1st and recurrent UTIs or acute pyelonephritis between January 1, 2014 and December 31, 2016 at the Jeju National University Hospital in Jeju, Korea.
Symptomatic UTIs in children present as unexplained fever ≥ 38℃, abnormal urinalysis results such as pyuria, and confirmed diagnosis of significant bacteriuria of ≥105 colony forming units of bacteria per milliliter (CFU/mL) in one urine specimen [13].
In addition to aseptic pyuria, patients with a suspected UTI on kidney ultrasound and dimercaptosuccinic acid (DMSA) kidney scan were included in this study, while patients with insufficient medical records were excluded from the study.
A urine sample was obtained via midstream collection in toilet-trained children or collected by a catheter or clean catch bag in children who were not toilet-trained. All children were assessed by blood tests (white blood cell, hemoglobin, creatinine, albumin, sodium, c-reactive protein, cystatin C), urine tests (urine protein-to-creatinine ratio, urine β2-microglobulin, urine N-acetyl-β-D-glucosaminidase), kidney ultrasonography (hydronephrosis, pelvic dilatation, increased renal parenchymal echogenicity, congenital anomalies (i.e. ureter duplication, ureterocele, renal agenesis, multicystic dysplastic kidney), and the DMSA kidney scan (renal cortical defect, functional uptake (%), right to left functional uptake ratio). For right to left functional ratio on the DMSA kidney scan, a value of 0.81–1.22 was considered to indicate symmetric kidneys, and values <0.81 or >1.22 were considered indicators of asymmetric kidneys. In cases of VUR, the degree of VUR was classified based on a grading system by the International Reflux Study in Children [14].
A statistical comparison was made between the results of the blood test, urine test, and imaging test regarding identification of VUR. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The results are expressed as mean±SD, and the t-test and ANOVA test were used to compare the mean. Linear regression analysis was performed for the prediction of VUR. Receiver operating characteristic curve and area under the curve (AUC) were used to compare the predictive strength of the variables, and P values of less than 0.05 were considered significant.
1. Ethics statement
The study protocol was approved by the institutional review board at Jeju National University Hospital (IRB file no. 2016-06-006).
Results
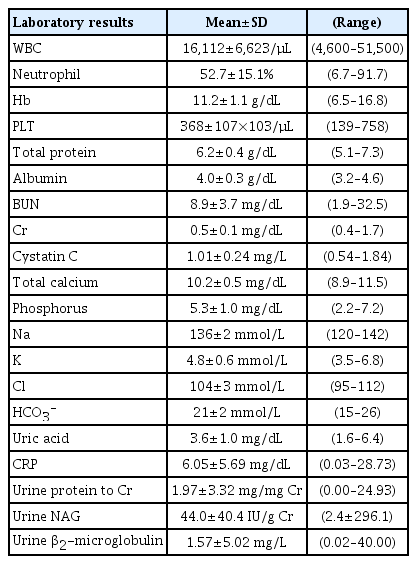
Among the 183 pediatric patients with a febrile UTI, 108 (59%) were male. The mean age was 10.8±18.0 (range, 0–115 months). Twelve patients (6.6%) had a history of UTIs and 2 (1.1%) had urological surgery. The mean duration of fever was 3.9±2.2 (range, 1–13 days) (Table 1). Laboratory findings were as follows: white blood cell (WBC), 16,112±6,623 (range, 4,600–51,500/mm3); segment neutrophil, 52.7±15.1 (range, 6.7–91.7%); albumin, 4.0±0.3 (range, 3.2–4.6 g/dL); blood urea nitrogen (BUN), 8.9±3.7 (range, 1.9–32.5 mg/dL); creatinine, 0.5±0.1 (range, 0.4–1.7 mg/dL); cystatin C, 1.01±0.24 (range, 0.54–1.84 mg/L); C-reactive protein (CRP), 6.05±5.69 (range, 0.03–28.73 mg/dL); and urine β2-microglobulin, 1.57±5.02 (range, 0.02–40.00 mg/L) (Table 2).
In our sample of 183 patients, urine cultures were identified in 148 patients (80.9%): E. coli in 131 patients (88.5%), Klebsiella sp. in 10 patients (6.8%), Enterobacter sp. in 4 patients (2.7%), and other bacteria in 3 patients (2.0%). Meanwhile, 10 patients (5.5%) were identified by positive blood culture: E. coli in 9 patients (90%) and Klebsiella sp. in 1 patient (10%). In all, 32 patients (21.6%) tested positive for extended-spectrum beta-lactamases (Table 3).
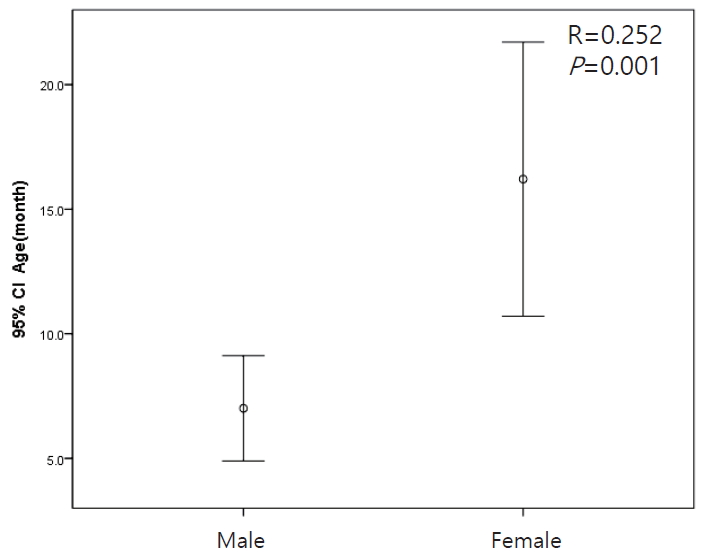
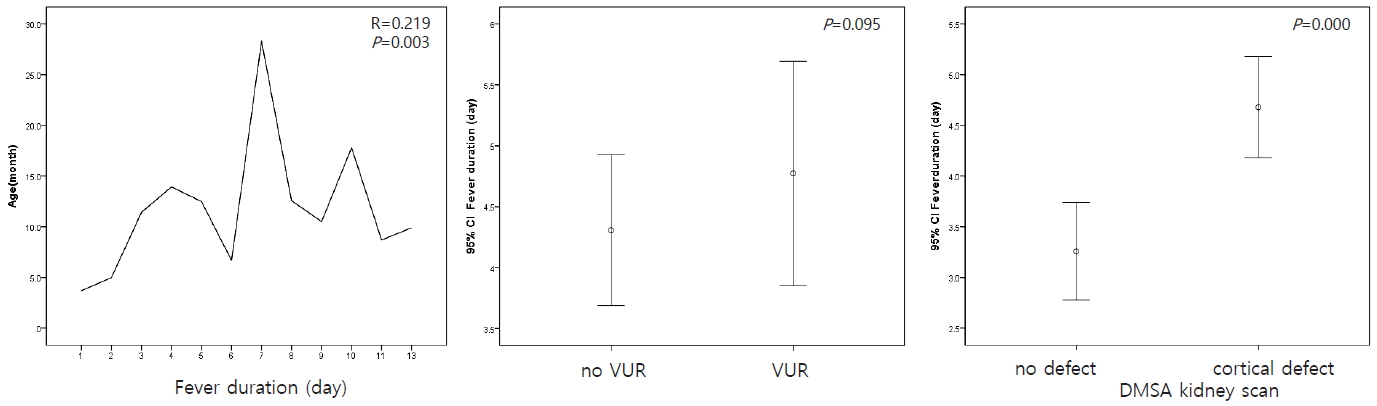
The mean age of males was lower than that of females (P=0.001) (Fig. 1). The duration of fever was higher in older children (P=0.003) and in patients with cortical defects on the DMSA kidney scan (P=0.000). However, the presence of VUR was not significantly associated with duration of fever (P=0.095) (Fig. 2).
All patients in our study underwent kidney ultrasonography. Increased parenchymal echogenicity in right and left kidneys on renal ultrasound was observed in 68 patients (37.2%) and 66 patients (36.1%), respectively. Hydronephrosis in right and left kidneys was diagnosed in 12 cases (6.6%) and 17 cases (9.3%), respectively. Also, renal agenesis in the right and left kidneys was diagnosed in 1 case (0.5%) and 1 case (0.5%), respectively. Additionally, neurogenic bladder and ureterocele were found in 1 patient (0.5%) and 1 patient (0.5%), respectively (Table 4).
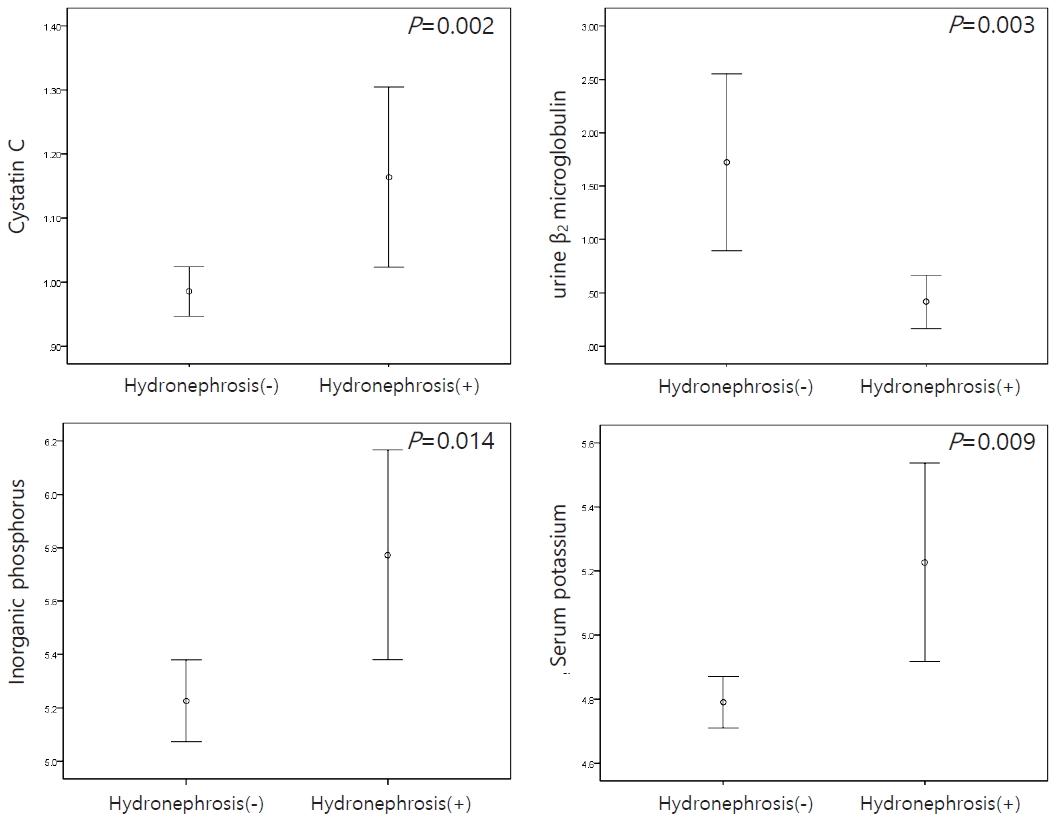
The laboratory results of patients with hydronephrosis revealed higher serum levels of cystatin C (P=0.002), phosphorus (P=0.014), and potassium (P=0.009) and a lower urine level of β2-microglobulin (P=0.003) than patients without hydronephrosis (Fig. 3).
DMSA kidney scan was performed in 152 patients. Their mean functional ratio was 51.0±6.6 in the right kidney and 49.0±6.6 in the left kidney. Unilateral cortical defects were detected in 76 patients (50%) and bilateral cortical defects in 14 patients (9.2%). Cortical defects were found in 48 patients (31.6%) in the right kidney and 56 patients (36.8%) in the left kidney. Right to left functional ratio was 1.1±0.8 (range, 0.3–1.0) (Table 5).
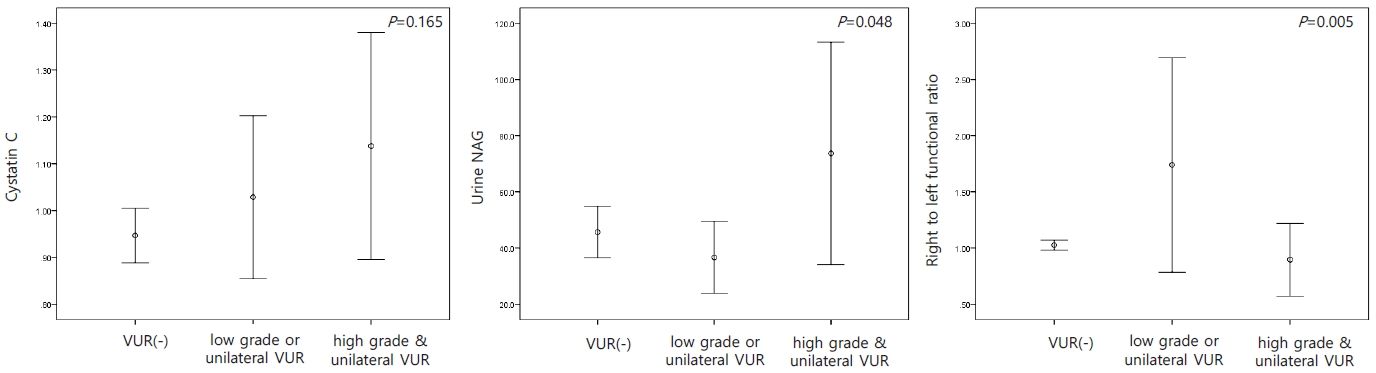
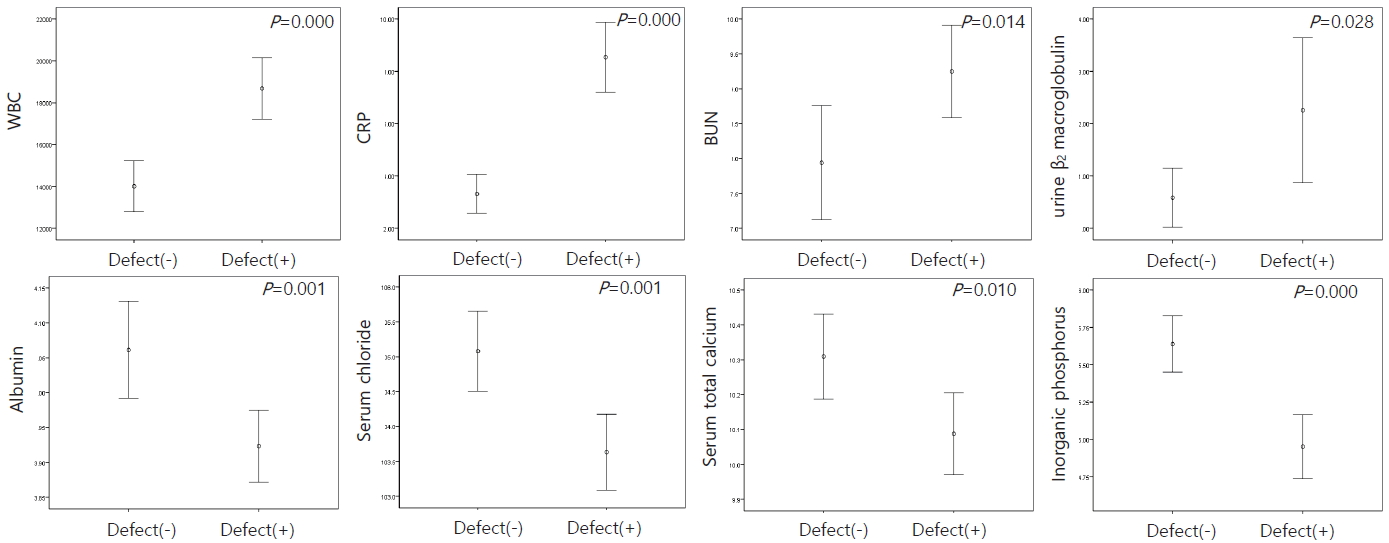
In patients with cortical defects on the DMSA scan, WBC (P=0.000), CRP (P=0.000), BUN (P=0.014), and urine β2-microglobulin (P=0.028) were significantly higher, while serum albumin (P=0.001), chloride (P=0.001), total calcium (P=0.010), and inorganic phosphorus (P=0.000) were significantly lower than in patients without cortical defects (Fig. 4). When comparing the asymmetric and symmetric groups on the DMSA scan, the levels of BUN (P=0.033), Cr (P=0.006), and CRP (P=0.003) were higher in the asymmetric cases than in the symmetric cases (Fig. 5).
Laboratory factors related to the presence of cortical defects on the DMSA kidney scan in urinary tract infections.
Laboratory factors related to asymmetric kidney on the DMSA kidney scan in urinary tract infections.
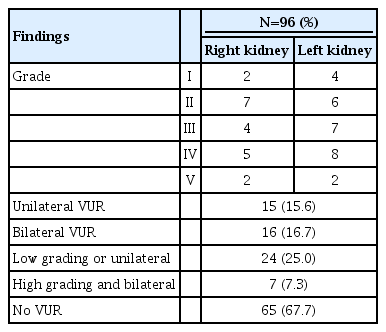
VCUG was performed in 96 patients. Among these, VUR was not detected in 65 patients (67.7%). Unilateral VUR was detected in 15 patients (15.6%) and bilateral VUR in 16 patients (16.7%). Low grade or unilateral VUR was detected in 24 patients (25.0%) and high grade and bilateral VUR in 7 cases (7.3%) (Table 6). The patients were categorized into 3 groups (no VUR, low grade or unilateral VUR, and high grade and bilateral VUR) and urine N-acetyl-β-D-glucosaminidase (NAG) (P=0.048) was higher in the high grade and bilateral VUR group than in the other groups. The right to left functional ratio was the highest in the group with low grade or unilateral VUR (P=0.005) (Fig. 6).
Linear regression analysis for the prediction of VUR was statistically significant for hydronephrosis in kidney ultrasound images (P=0.000) or cortical defects (P=0.000) or asymmetric kidney (P=0.049) on the DMSA kidney scans (Table 7). The most significant predictors of high grade and bilateral VUR were the presence of hydronephrosis in kidney ultrasound images and cortical defects on the DMSA kidney scan, followed by hydronephrosis only identified using the AUC (Fig. 7).
Discussion
Hydronephrosis and VUR are well-known risk factors for UTIs in children [15-18]. In a retrospective single center study on the incidence of UTIs in infants with antenatal diagnosis of hydronephrosis, 24 (13%) in 192 patients experienced at least one episode of a UTI during the follow-up period. Among these patients with a UTI, high grade VUR was detected in 15 patients (63%) [19]. Indeed, a different study also suggested that prenatal hydronephrosis with high grade VUR may pose as a risk factor for UTIs [20].
Kidney sonography can be used to predict VUR in neonates and infants [21-24]. In a study on 735 infants diagnosed with mild hydronephrosis, 189 infants (25.7%) had VUR. This study also included patients with UTIs, and there was no statistically significant relationship between mild hydronephrosis and the presence of a UTI. However, UTI incidence was significantly higher in patients with VUR grade 3 or higher than in those with a lower grade VUR, regardless of the presence of hydronephrosis during the follow-up period [24]. According to another study published in 2008, studies in infants under 3 months of age also found that kidney sonography was statistically significant in predicting the presence of VUR in high grade VUR children [25].
Alternatively, ultrasonography was used as a screening test for genitourinary anomalies in patients with a UTI [26,27]. According to Nelson et al. [27], 389 (17.2%) in 2,259 patients who performed kidney ultrasonography were diagnosed with hydronephrosis. Mild hydronephrosis was detected in 298 patients (13.2%) and high grade hydronephrosis in 91 patients (4.0%). However, ultrasonography screening of genitourinary anomalies such as VUR had low sensitivity and specificity [27].
To evaluate UTI patients by imaging, we used “Top-down approach”, which focuses on identifying children at risk for pyelonephritis or renal scarring, whether or not VUR is present, performing a DMSA scan during the acute inflammatory phase of UTI. Once renal involvement is demonstrated on the DMSA scan, the patient then undergoes VCUG to find out VUR. Using this method will help reduce unnecessary invasive test of VCUG [28,29].
In this study, VCUG was performed in 52% of UTI patients, 32.2% of patients with reflux, and only 7 patients (3.8%) with bilateral or high grade VUR who may need prophylatic antibiotics or urosurgery. Therefore, it is important to find the predictive marker of VUR for selective evaluation.
There are several studies on the prediction of VUR in patients with UTIs showing cortical defects and relative uptake on the DMSA scans [30,31]. In the Hong et al. [30], study, the sample was divided into groups with and without VUR. Although the patients in the group with VUR were older, there was no significant difference between the participants in the control group and VUR group regarding sex, fever duration, WBC count, CRP, and estimated glomerular filtration ratio. In the group with VUR, the asymmetric relative uptake was more frequently observed on the DMSA kidney scan. There were no significant differences between the 2 groups in terms of the presence of hydronephrosis, cortical defects on the DMSA kidney scan, and hydronephrosis with simultaneous cortical defect. The sensitivity and specificity of cortical defects on the DMSA kidney scan, suggestive of VUR, were 79% and 5%, respectively. The sensitivity and specificity of hydronephrosis in ultrasound images were 39% and 70%, respectively [30].
Snodgrass et al. [32], performed a cross-sectional observational study in which VUR was diagnosed in 15.5% of the 565 patients with cortical defects on the DMSA kidney scans. Additionally, higher grades of VUR have been associated with higher risks of cortical defects on the DMSA kidney scans. Furthermore, UTI recurrence was more frequent in patients with a higher grade of VUR [32]. Another retrospective study on a pediatric population found that older females (>27 months) with UTIs had more renal scars on the DMSA kidney scans than younger females. Additionally, renal scars were more common in patients with high grades of VUR (IV and V) than in patients with low grades of VUR. However, renal scars in patients with UTIs were not significantly different among unilateral and bilateral VURs [33].
Laboratory findings of patients with UTIs in our study were related to inflammatory responses and correlated with cortical defects on the DMSA kidney scan. The severity of the inflammation should be interpreted as a correlation with the cortical defect [34], such as acute pyelonephritis. Therefore, it is difficult to predict the presence of VUR based on the severity of the inflammatory response. If hydronephrosis is diagnosed in renal ultrasonography in patients with UTIs, the possibility of VUR should be considered [18,21,24]. In addition, if UTIs are present in patients with hydronephrosis, cortical defects on the DMSA kidney scans are strongly suggestive of VUR [30,31]. Therefore, in such cases, VCUG should be performed [35-38].
Recently, some new biomarkers predicted renal scarring in children with VUR, which included urinary level of neutrophil-gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and liver-type fatty-acid-binding protein (L-FABP). The median value of uNAGL/Cr and uL-FABP/Cr was higher in groups with VUR than in groups without VUR, and the median value of uKIM-1/Cr was similar in both the groups [39]. According to a paper published in 2013, there are reports in the literature of urinary tissue inhibitor of metalloproteinase-1 (TIMP1) and matrix metalloproteinase 9 (MMP9) levels being used as predictors of VUR in neonates diagnosed with prenatal hydronephrosis. Indeed, MMP9, MMP9/Cr, MMP9/TIMP1, and MMP9/TIMP1/Cr ratios were higher in patients with VUR than in those without VUR. Based on these results, the authors discussed the use of screening tests for the presence of VUR in neonates with hydronephrosis to reduce their exposure to radiation, as in VCUG [40]. Other studies suggests that MMP9 and TIMP1 could be used as biomarkers to predict renal scars in children with UTIs [41,42].
The limitation of our study was inclusion criteria including children with 1st attack of UTI as well as with recurrence of UTI. Another limitation was that the definition of UTI according to age based on 12 months of age is not introduced. In our study, children with febrile UTI were retrospectively included as a symptomatic UTI regardless of age.
In conclusion, the presence of hydronephrosis in kidney ultrasound or cortical defects or asymmetric kidney on the DMSA kidney scans can be predictive markers for VUR. Our study can help to minimize exposure to radiation through the selective implementation of VCUG.
Acknowledgements
This study was supported by a research grant from the Jeju National University Hospital development fund in 2016.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.