| Child Kidney Dis > Volume 27(2); 2023 > Article |
|
Abstract
Purpose
To analyze electrocardiograms (ECGs) of patients with a salt-losing tubulopathy (SLT) and to determine the frequency and risk factors for long QT and arrhythmia.
Methods
A total of 203 patients aged <19 years with SLT, specifically Bartter syndrome and Gitelman syndrome, who had a 12-lead ECG were included in this retrospective study. We analyzed the presence of an arrhythmia or prolonged corrected QT (QTc) on ECGs obtained for these patients. Demographic and laboratory data were compared between patients with abnormal and normal ECG findings.
Results
Out of the 203 SLT patients, 38 (18.7%) underwent electrocardiography and 10 (40.0%) of 25 patients with inherited SLT had abnormal ECG findings, including prolonged QTc and arrhythmias. The abnormal ECG group had significantly lower serum potassium levels than the normal group (median [interquartile range]: 2.50 mmol/L [2.20ŌĆō2.83] vs. 2.90 mmol/L [2.70ŌĆō3.30], P=0.036), whereas other serum chemistry values did not show significant differences. The cutoff level for a significant difference in QTc interval was serum potassium level <2.50 mmol/L. One cardiac event occurred in a 13-year-old boy, who developed paroxysmal supraventricular tachycardia and underwent cardiac ablation. No sudden cardiac deaths occurred in this cohort.
Bartter syndrome (BS) refers to an inherited salt-losing tubulopathy (SLT) characterized by a defect in salt reabsorption in the thick ascending limb of the loop of Henle [1]. Gitelman syndrome (GS) shares metabolic abnormalities with BS [2] and is caused by a defect in the thiazide-sensitive sodium/chloride cotransporter in the distal convoluted tubule, resulting in SLT [3]. These conditions may result in hypokalemia, metabolic alkalosis, activated plasma renin, and normal or low blood pressure [4]. Fluctuations in potassium levels can significantly affect the electrical characteristics of the heart muscles. Hypokalemia can lead to the development of ventricular arrhythmias, resulting in life-threatening conditions. Hypokalemia also causes electrocardiographic changes, such as QT interval prolongation, ST-segment depression, flat or low-amplitude T waves, and often prominent U waves [5]. Patients may experience presyncope, vertigo, ataxia, or blurred vision [6-8].
The frequency of cardiac arrhythmia or sudden death is unknown. To date, cardiac arrhythmia or death associated with BS and GS has been reported in six cases in the literature: three as cardiac arrhythmia and three as sudden cardiac death [7-11]. Therefore, the cardiac manifestations of BS and GS can be fatal. Hypokalemia, hypomagnesemia [7], QT dispersion, and JT dispersion [12] have been suggested as risk factors for cardiac arrhythmia in SLT. However, no large-scale studies have been conducted to date.
Therefore, the purpose of this study was to assess the frequency of electrocardiographic abnormalities and identify associated risk factors in patients diagnosed with inherited SLT.
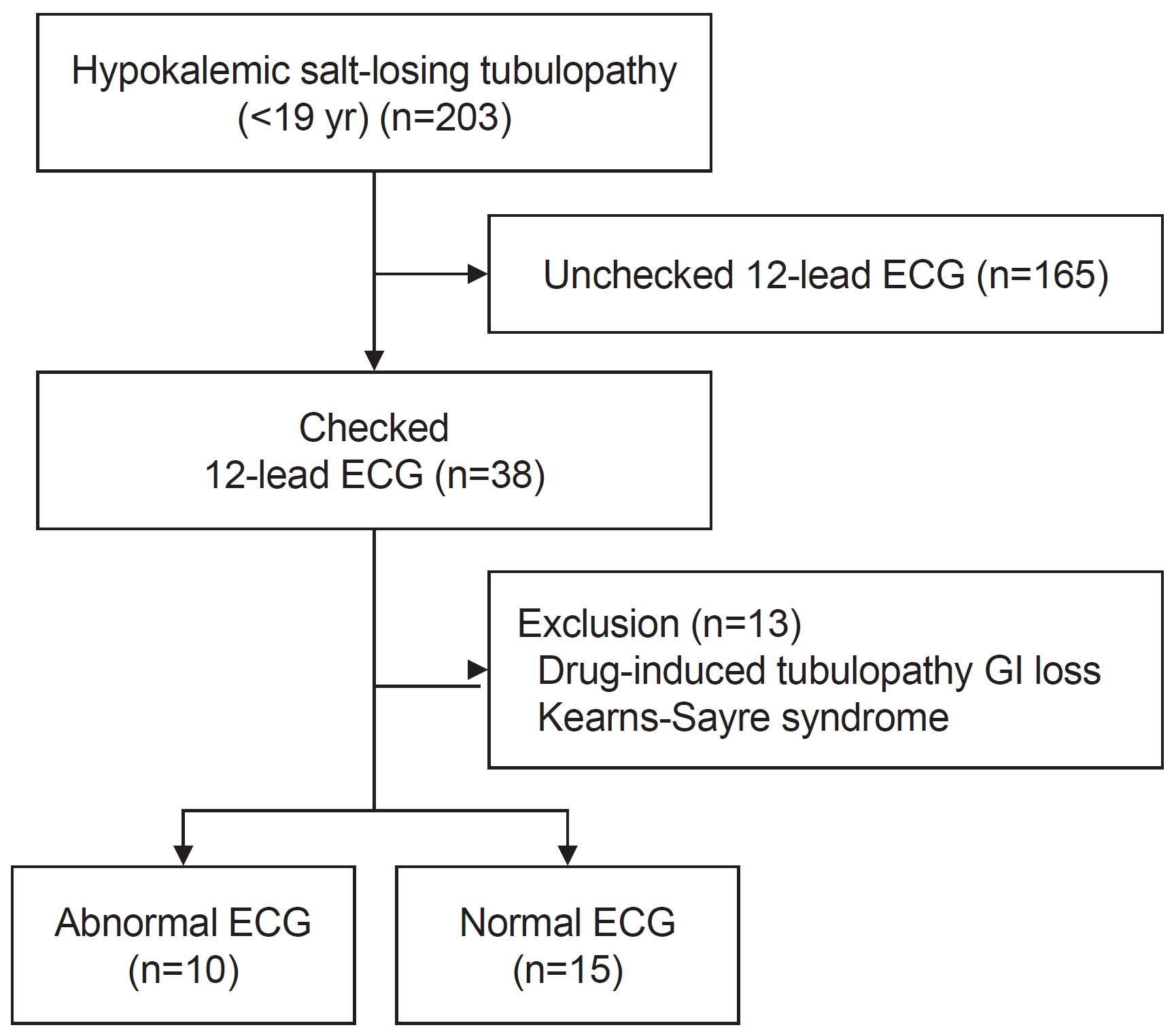
Patients visiting Seoul National University ChildrenŌĆÖs Hospital between January 1991 and May 2022 who were younger than 19 years of age and who underwent 12-lead electrocardiography with a diagnosis of an SLT were included in this retrospective study. Inherited SLT encompassed BS and GS. The exclusion criteria included non-nephrogenic electrolyte imbalance and other genetic diseases (Fig. 1).
All electrocardiograms (ECGs) obtained for eligible patients during the study period were analyzed. QT intervals were measured in leads II, V5, and V6, and the longest value was used. The QT interval was adjusted for heart rate using the Bazett formula (QTc=QT/RR0.5; all units are ms). ECGs findings were categorized as abnormal if they included a prolonged corrected QT (QTc) or an abnormal rhythm. Abnormal rhythm was defined as the absence of sinus rhythm. All patientsŌĆÖ data of QTc were classified based on recommended QTc interval value from Goldenberg and Moss [13]. For classification, those with a prolonged QTc were grouped in the abnormal ECG group, whereas those with normal and borderline QTc values were grouped into the normal ECG group. For group comparisons, one ECG per patient was selected, with the criterion being an abnormal rhythm or the longest QTc.
Clinical information, including sex, age, height, weight, and body mass index (BMI), as well as serum sodium, potassium, calcium, and magnesium levels, were compared between the two groups. Age, height, weight, and BMI were analyzed closest to the time of ECG measurement, and laboratory findings including serum electrolyte and creatinine levels were analyzed within 24 hours of ECG measurement.
Statistical analyses were performed using SPSS version 27.0 (IBM Corp.). Continuous variables are presented as medians (interquartile range [IQR]). Between-group comparisons were conducted using the Mann-Whitney U test for continuous variables and the Fisher exact test for categorical variables. Statistical significance was set at a P-value below 0.05.
A total of 203 patients were diagnosed with SLT at Seoul National University ChildrenŌĆÖs Hospital during the study period. Among them, 38 (18.7%) had available ECG data. Patients with non-nephrogenic tubulopathies such as chemotherapy-induced tubulopathy, electrolyte imbalance derived from the gastrointestinal tract, and other known genetic diseases were excluded from the study (n=13). Ultimately, the study group comprised 25 individuals.
The number of ECGs performed varied for each patient (median, 2; range, 1ŌĆō127). A total of 172 ECGs were obtained from eligible patients, of which 80 were classified as normal and 92 as abnormal. One patient had 127 ECGs (49 normal, 78 abnormal) alone due to a paroxysmal supraventricular tachycardia (PSVT) event and prolonged hospitalization; therefore, we excluded this patient. Thereafter, there were 31 normal ECGs and 14 abnormal ECGs. ECGs were mostly performed due to hypokalemia or symptomatic conditions such as poor oral intake, diarrhea, vomiting, and muscle cramps; besides these conditions, ECGs were also performed before the thiazide loading test to diagnose SLT (n=4) and for pre-operative evaluation (n=3).
To compare the characteristics and biochemical profiles of patients according to ECGs, one ECG was assigned to each patient. This resulted in 15 patients, including one patient with PSVT in the normal ECG group and 10 patients in the abnormal ECG group (Fig. 1). No significant differences were noted between the normal and abnormal ECG groups in terms of sex, age, weight, height, or BMI (Table 1). A comparison of serum chemistry values is shown in Table 1. The median serum potassium levels were 2.90 mmol/L (IQR, 2.70ŌĆō3.30 mmol/L) in the normal ECG group and 2.50 mmol/L (IQR, 2.20ŌĆō2.83 mmol/L) in the abnormal ECG group, showing a significant difference between the two groups (P=0.036). No significant differences were noted in the serum sodium, chloride, total calcium, magnesium, or total carbon dioxide levels between the two groups.
We also compared the general features and biochemical values based on serum potassium levels (Tables 2, 3). When comparing serum potassium levels below 3.00 mmol/L and above 3.00 mmol/L, no statistical significance was noted for any indicator (Table 2). However, when comparing serum potassium levels below 2.50 mmol/L and above 2.50 mmol/L, a significant difference was noted in the QTc interval. Between the group with a serum potassium level Ōēź2.50 mmol/L (n=19) and the group with a level <2.50 mmol/L (n=6), the median QTc interval was 435.0 ms (IQR, 403.0ŌĆō468.0 ms) and 491.0 ms (IQR, 439.5ŌĆō586.8 ms), respectively, showing a significant difference (P=0.030).
One patient presented with PSVT (Fig. 2); he was a 13-year-old boy with severe hypokalemia (2.20 mmol/L) and normal serum magnesium (1.70 mmol/L). The patient's father had a history of sudden cardiac death of an unknown cause. The patient underwent cardiac ablation. The patient harbored a confirmed CLCNKB mutation and was diagnosed with type III BS. He was the only patient who underwent a cardiac intervention for PSVT, specifically cardiac ablation. To date, PSVT has not recurred; however, due to recurrent severe hypokalemia, long-term inpatient treatment was administered, including potassium replacement and other medications, including potassium-sparing diuretics and a thiazide, with regular electrolyte level and ECG monitoring.
Fourteen patients (56.0%) were currently being followed up in the nephrology or pediatric departments at this center, but the remaining patients were either transferred (n=4) or lost to follow-up (n=7). Only two individuals had normalized QTc, three still had prolonged QTc, and five had no follow-up ECG; therefore, normalization was unknown. Three patients progressed to chronic kidney disease with a long-term prognosis regardless of the ECG findings. No sudden cardiac death was observed in any patient during the study period.
This retrospective study assessed the frequency of electrocardiographic abnormalities and explored the associated risk factors in patients diagnosed with inherited SLT. The study findings revealed that 40.0% of the patients with SLT had a prolonged QTc interval, and one of the 25 patients experienced a cardiac arrhythmia in the form of PSVT. The incidence of prolonged QTc is not well established in BS [1], whereas it can be as high as 50.0% in GS [3].
Cortesi et al. [7] suggested that SLT itself increases patientsŌĆÖ vulnerability to arrhythmia, especially in case of severe hypokalemia. Baseline ECG checks should be conducted for patients with BS and GS [14]. However, the rate of ECG screening for SLT was found to be low (18.7%) in our study cohort. Therefore, it is important to enforce regular ECG screening to enable early detection of any potential arrhythmia or QTc prolongation in patients with SLT. We found significant differences in serum potassium levels between the normal and abnormal ECG groups, indicating an association between serum potassium levels and prolonged QTc or arrhythmia. This aligns with the findings of previously reported cases that consistently emphasized the association between hypokalemia and occurrence of cardiac arrhythmia or death in individuals with BS and GS [7-11]. The target serum potassium level of 3.00 mmol/L for the general population may not be applicable to patients with SLT. According to Konrad et al. [1] and Blanchard et al. [3], maintaining a serum potassium level of 3.00 mmol/L may require an increased dose of potassium supplements and other medications, possibly leading to a decrease in medication compliance in patients with severe SLT. Consistent with a previous report [7], the present study analyzed the potassium cutoff level for QTc prolongation, which was a serum potassium level of 2.50 mmol/L. A prolonged QT interval is known to increase the risk of developing ventricular arrhythmias [13]. Therefore, achieving and maintaining this target serum potassium level in SLT is crucial to avoid arrhythmia.
The recommended treatment to maintain an optimum serum potassium level is as follows. A potassium supplement containing potassium chloride must be administered, starting with 1ŌĆō2 mmol/kg/day, and titration must be tailored to the individual patient while considering symptoms and side effects. In the case of severe hypokalemia, intravenous supply is essential. Simultaneously, the patients are strongly encouraged to consume potassium-rich foods [1,2]. Patients with BS are usually also administered nonsteroidal anti-inflammatory drugs (NSAIDs), such as indomethacin (1ŌĆō4 mg/kg/day divided in 3ŌĆō4 doses), ibuprofen (15ŌĆō30 mg/kg daily in 3 doses), and celecoxib (2ŌĆō10 mg/kg/day divided in 2 doses), and there is insufficient evidence to recommend specific NSAIDs. In addition, for abnormal electrolyte status, potassium-sparing diuretics, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and thiazides may be helpful, but their routine use is not recommended, as they may result in hypovolemia [1]. In the case of GS patients, indomethacin is rarely used, and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are occasionally used, but should be discontinued if there is a risk of hypovolemia [2].
This study has some limitations. First, while the number of patients diagnosed with SLT was relatively high, the low number of ECGs available for patients resulted in limited patient recruitment. In the future, more ECGs should be performed to increase the statistical power. Second, the number of ECGs and intervals varied widely among individuals, making it statistically challenging to analyze each ECG individually. We also evaluated ECG abnormalites other than prolonged QTc, such as ST depression, flat T-wave, and prominent U wave. However, since those abnormalities were only identified in a small number of patients, we based our comparison on QTc prolongation alone. In the presence of abnormal ECG findings, regular ECG follow-ups are necessary for assessing risk factors. Third, because of the retrospective nature of this study, all patients had different follow-up periods; therefore, it was not possible to compare long-term outcomes. Lastly, this study does not provide evidence to determine whether BS and GS themselves increase the risk of arrhythmias or if the higher incidence of arrhythmias is solely due to hypokalemia.
In conclusion, as hypokalemia is strongly correlated with prolonged QTc, it is important to maintain serum potassium levels above 2.50 mmol/L and to be aware of symptoms such as acute diarrhea or vomiting, which can cause hypokalemia. As the incidence of abnormal ECG findings was found to be quite high (40.0%) in our study, it may be beneficial to conduct ECG screening for every BS and GS patient. If an abnormality is found during ECG screening, regular follow-up ECGs are required, and further cardiologic evaluation should be considered.
Notes
Ethical statements
This study was approved by the Institutional Review Board of the Seoul National University Hospital (IRB No. H-2306-115-1439) and complied with the principles of the Declaration of Helsinki. The informed consent was waived because of the retrospective nature of this study.
Conflicts of interest
Hee Gyung Kang is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
References
1. Konrad M, Nijenhuis T, Ariceta G, Bertholet-Thomas A, Calo LA, Capasso G, et al. Diagnosis and management of Bartter syndrome: executive summary of the consensus and recommendations from the European Rare Kidney Disease Reference Network Working Group for Tubular Disorders. Kidney Int 2021;99:324-35.


2. Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 1966;79:221-35.

3. Blanchard A, Bockenhauer D, Bolignano D, Calo LA, Cosyns E, Devuyst O, et al. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2017;91:24-33.


4. Zelikovic I. Hypokalaemic salt-losing tubulopathies: an evolving story. Nephrol Dial Transplant 2003;18:1696-700.


6. Bettinelli A, Tosetto C, Colussi G, Tommasini G, Edefonti A, Bianchetti MG. Electrocardiogram with prolonged QT interval in Gitelman disease. Kidney Int 2002;62:580-4.

7. Cortesi C, Lava SA, Bettinelli A, Tammaro F, Giannini O, Caiata-Zufferey M, et al. Cardiac arrhythmias and rhabdomyolysis in Bartter-Gitelman patients. Pediatr Nephrol 2010;25:2005-8.

9. Malafronte C, Borsa N, Tedeschi S, Syren ML, Stucchi S, Bianchetti MG, et al. Cardiac arrhythmias due to severe hypokalemia in a patient with classic Bartter disease. Pediatr Nephrol 2004;19:1413-5.


10. Nakane E, Kono T, Sasaki Y, Tokaji Y, Ito T, Sohmiya K, et al. Gitelman's syndrome with exercise-induced ventricular tachycardia. Circ J 2004;68:509-11.


11. Scognamiglio R, Negut C, Calo LA. Aborted sudden cardiac death in two patients with Bartter's/Gitelman's syndromes. Clin Nephrol 2007;67:193-7.


Table┬Ā1.
Comparison of patient characteristics and biochemical values
Table┬Ā2.
Comparison of characteristics and biochemical values by potassium level (Ōēź3.0 mmol/L vs. <3.0 mmol/L)
Table┬Ā3.
Comparison of characteristics and biochemical values by potassium level (Ōēź2.5 mmol/L vs. <2.5 mmol/L)
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 856 View
- 12 Download
- ORCID iDs
-
Seong Ryeong Kang

https://orcid.org/0009-0001-9980-3940Yo Han Ahn

https://orcid.org/0000-0002-8185-4408Hee Gyung Kang

https://orcid.org/0000-0001-8323-5320Naye Choi

https://orcid.org/0000-0003-2966-9608 - Related articles