| Child Kidney Dis > Volume 26(2); 2022 > Article |
|
Abstract
The messenger RNA-based vaccine for the coronavirus disease 2019 (COVID-19) may induce glomerulonephritis, including immunoglobulin A nephropathy (IgAN). New-onset IgAN triggered by vaccination against COVID-19 has been reported rarely, especially in children. Herein, we report a pediatric case of newly diagnosed IgAN after administration of the Pfizer vaccine for COVID-19. A 12-year-old girl was referred to our hospital for evaluation of gross hematuria after inoculation with the second dose of PfizerŌĆÖs COVID-19 vaccine; she had no adverse effects after the first dose. At the time of admission, she showed heavy proteinuria and persistent hematuria. Kidney biopsy revealed an IgAN, and she was treated with an oral steroid and an angiotensin-converting enzyme inhibitor. Four months after discharge, the proteinuria and hematuria resolved completely.
Immunoglobulin A (IgA) nephropathy (IgAN) is the most common type of glomerulonephritis in children globally, especially in Asian and European countries [1]. The coronavirus disease 2019 (COVID-19) is a global health problem that has spread from Wuhan city in China to places around the world since December 2019. The pathogen responsible for this, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can damage the kidney and result in acute kidney injury (AKI) or acute tubular necrosis as well as a respiratory compromise [2]. After the U.S. Food and Drug Administration approved the use of the Pfizer COVID-19 vaccine for children in 2021, a few cases of IgAN flare up or IgAN newly diagnosed after vaccination have been reported [3]. However, most of the newly diagnosed IgAN cases have been reported in adults [3]. Herein, we report a rare case of a pediatric patient with gross hematuria and newly diagnosed IgAN after administration of the Pfizer vaccine.
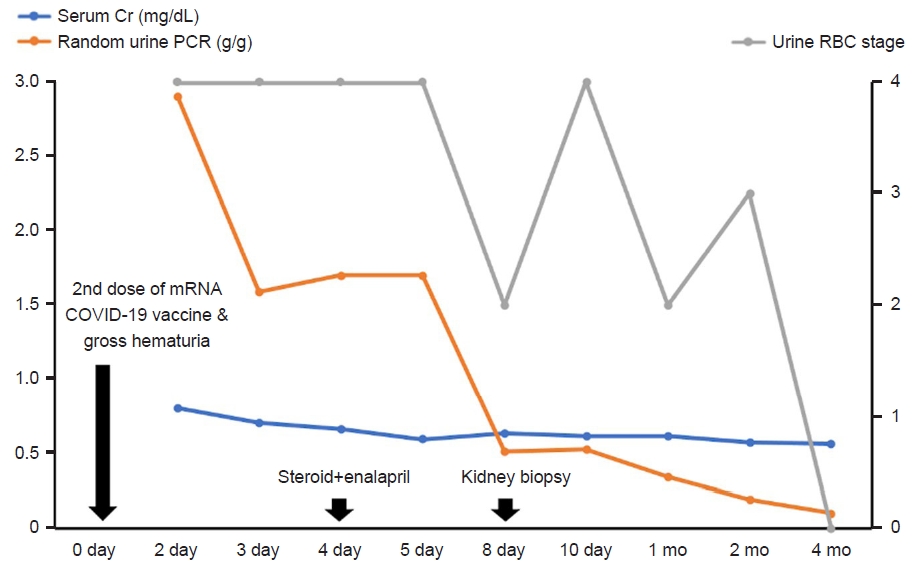
A 12-year-old girl was referred to our emergency department with gross hematuria and heavy proteinuria (random urine protein-to-creatinine ratio [PCR] of 4.94 g/g, normal <0.2 g/g) after the second dose of the messenger RNA (mRNA)-based COVID-19 vaccine (Pfizer). She had no hematuria after the first dose but developed dark-brown-to-red hematuria several hours after administration of the second dose of the vaccine 2 days prior to admission. The gross hematuria disappeared the next day; the patient had no fever or urinary symptoms except for hematuria, abdominal pain, and symptoms of upper respiratory infection over the previous 4 weeks. She denied performing strenuous exercise or experiencing recent trauma. Her initial vital signs were as follows: blood pressure of 137/80 mmHg (systolic >95th percentile +12 mmHg, diastolic >95th percentile), pulse rate of 90 beats/min, respiratory rate of 16 breaths/min, body temperature of 37.2┬░C, and oxygen saturation of 99% for room air. Her body weight was 55.2 kg (86th percentile) and her height was 150.1 cm (29th percentile), with a body mass index of 24.5 kg/m2 (95th percentile). Physical examination revealed no specific findings, and pretibial pitting edema was not obvious. Initial urinalysis showed severe microscopic hematuria (>60 red blood cells/high power field [RBCs/HPF]) and heavy proteinuria (urine PCR 2.9 g/g). Her 24-hour urine protein level was 2,455 mg/day (71.8 mg/m2/hr). The dysmorphic RBC content in the urine was 43% of the total RBC. The urine myoglobin level was mildly elevated at 91.7 ng/mL (reference: 0ŌĆō25 ng/mL). The initial blood test results were as follows: blood urea nitrogen=15.5 mg/dL, creatinine=0.8 mg/dL, aspartate aminotransferase=20 U/L, alanine aminotransferase=9 U/L, protein=7.2 g/dL, albumin=4.4 g/dL, total cholesterol=246 mg/dL, myoglobin <21 ng/mL (reference: 25ŌĆō72 ng/mL), C-reactive protein=0.49 mg/dL, procalcitonin=0.14 ng/mL (reference: <0.15 ng/mL), IgA=357 mg/dL (reference: 42ŌĆō295 mg/dL), C3=149 mg/dL (normal: 75ŌĆō175 mg/dL), and C4=17.6 mg/dL (normal: 14ŌĆō40 mg/dL). On the 3rd day of hospitalization, her serum protein and albumin levels decreased (6.2 and 3.5 g/dL, respectively), and the cholesterol level remained persistently high (218 mg/dL). The creatine phosphokinase (CPK) level was 257 U/L (reference: 26ŌĆō192 U/L), and urine myoglobin was normalized (<21 ng/mL). We started steroid (deflazacort) therapy at a dose of 1 mg/kg/day and an angiotensin-converting enzyme (ACE) inhibitor (enalapril) at 0.1 mg/kg/day owing to suspicion of acute glomerulonephritis. We then conducted a kidney biopsy to confirm the diagnosis on the 6th day of admission. Microscopic findings from the kidney biopsy revealed IgAN as shown in Fig. 1; there were hypercellular glomeruli (<50%) involving mesangial cells (hematoxylin and eosin staining) and moderate amounts of mesangial deposits (electron microscopy finding). Cellular crescents were found in five glomeruli (21%), and the epithelial cells showed slight focal effacement of the foot processes. The tubules showed moderate focal atrophy and loss with infiltration of mononuclear cells, together with mild interstitial fibrosis. Immunofluorescence microscopy showed mesangial staining of predominant IgA (3+), C3 (1+), and kappa (2ŌĆō3+). Therefore, we confirmed our diagnosis of IgAN grade II (H.S. Lee's glomerular grading system). On the 10th day of admission, the serum creatinine and urine PCR levels were reduced to 0.61 mg/dL and 0.52 g/g, respectively; she was then discharged from the hospital. While the microscopic hematuria persisted, the urine PCR continued to decrease, with a value of 0.34 g/g approximately 1 month after discharge; the total cholesterol level at this time was 237 mg/dL. She had no hematuria or proteinuria (urine PCR 0.1 g/g) at 4 months after discharge from the hospital, and the total cholesterol level had decreased to 211 mg/dL. We gradually tapered off the steroid dose (0.4 mg/kg/day). The clinical course is presented in Fig. 2.
This is a case report of pediatric IgAN presenting with gross hematuria and heavy proteinuria related to administration of the second dose of the mRNA-based COVID-19 vaccine. To the best of our knowledge, this is the first pediatric case report in Korea. Similar to other respiratory viruses, infection with the SARS-CoV-2 pathogen, which has become a global pandemic, can cause nephropathy in children [4]. This condition is mostly represented by tubular-interstitial nephritis, AKI, proteinuria, and hematuria [2]. However, the vaccine for SARS-CoV-2 can also induce glomerulonephritis in children [5]. According to a study by Klomjit et al. [6], IgAN is the most common kind of glomerulonephritis related to the mRNA-based COVID-19 vaccine. Globally, this is a rare case report till date.
The pathogenesis of IgAN has a multihit hypothesis. The production of galactose-deficient IgA1 in the hinge region contributes to revelation of N-acetylgalactosamine glycan, which is highly antigenic [7]. The galactose-deficient IgA1 combines with the autoantibody and forms circulatory immune complexes in patients with IgAN who lack mucosal immunity. Then, it is deposited in the renal glomerular mesangium and causes IgAN. Other pathophysiological mechanisms include inflammatory cascade, such as cytokines, chemokines, and activation of the complement pathway stimulated by immune complexes [7]. With respect to COVID-19 infection, Bitencourt et al. [8] have suggested that SARS-CoV-2 can directly cause injury to the renal tubular cells and podocytes via binding to the ACE2 on the cellular membrane. Direct cell damage can disturb the clearance of angiotensin II, leading to dysregulation of the renin-angiotensin system [8]. In addition, through response to viral infection, the cytokine storm can recruit leukocytes and neutrophils, ultimately causing endothelial injury and AKI [2]. Therefore, it is plausible that SARS-CoV-2 can induce renal glomerular and tubular injuries by different mechanisms. Serafinelli et al. [4] reported two different cases of biopsy-proven acute tubulointerstitial nephritis in a 12-year-old girl and a second kidney-biopsy-proven crescentic glomerulonephritis in a 10-year-old girl with previously diagnosed HenochŌĆōSchonlein purpura nephritis related to SARS-CoV-2 infection. Nevertheless, the mechanism by which the mRNA-based COVID-19 vaccine involves the kidney is not yet obvious [3]. In a study by Wisnewski et al. [9] where four subjects were vaccinated with the Pfizer or Moderna vaccine and had never had COVID-19 before, the SARS-CoV-2 spike antigen-specific serum IgA and IgG increased geometrically to peak levels at approximately 20 and 7 days after the first and second doses of the mRNA-based vaccine, respectively, and persisted for 100 days or more. Agrati et al. [10] found that all naive COVID-19 subjects showed significant elevations of the anti-receptor-binding domain antibodies after the first and second doses of the Pfizer mRNA vaccine (BNT162b2). Furthermore, the production of T helper 1 cytokines, such as interferon-╬│, tumor necrosis factor-╬▒, and interleukin 2 in spike-specific T cells, increased significantly [10]. In accordance with these results, the enhancement of the circulating IgA, B, and T-cell responses after mRNA-based COVID-19 vaccination might affect the glomerulus and cause IgAN, as in our case. Klomjit et al. [6] also reported new cases and relapse of glomerulonephritis after mRNA-based COVID-19 vaccination. IgAN was the most common type among patients after vaccination. This finding might be attributed to the fact that IgA involves major immune responses after mRNA-based COVID-19 vaccination. Udagawa and Motoyoshi [11] also reported that relapse and flare up of adolescent IgAN could occur after vaccination even during remission. Horino et al. [5] and Kudose et al. [12] reported newly diagnosed cases of IgAN following vaccination for COVID-19 in children and an adult, respectively.
In case reports of renal complications after COVID-19 vaccination, most patients presented with fever and gross hematuria within 1 or 2 days after administration of the Pfizer mRNA COVID-19 vaccine and accompanied by proteinuria [3]. Abdel-Qader et al. [13] reported the case of a 12-year-old boy with IgAN who developed gross hematuria and heavy proteinuria (1.7 g/day) within 24 hours after the first dose of the Pfizer COVID-19 vaccine. Our patient showed gross hematuria and proteinuria on the day following the second dose of mRNA-based COVID-19 vaccination, and IgAN was newly diagnosed. The development of rhabdomyolysis was monitored initially because the patient had mild myalgia, macroscopic hematuria with laboratory findings of mildly elevated serum CPK, and urine myoglobin. However, the serum CPK did not exceed 1,000 U/L. In addition, the serum CPK and urine myoglobin levels resolved to the normal ranges within 2 days after intravenous fluid therapy. Notably, rhabdomyolysis has been reported not only in COVID-19 infection but also as a complication of vaccination for COVID-19 [14]. Nassar et al. [14] reported mRNA-based COVID-19 vaccine-related rhabdomyolysis in a 21-year-old male with a CPK of 22,000 U/L and presumed that it could be mediated by cytokine storm and direct viral invasion.
There have been reports of pediatric IgAN after COVID-19 vaccination. Horino et al. [5] reported the case of a 17-year-old male with de novo IgAN induced by the second dose of Pfizer vaccination. Hematuria (>100 RBCs/HPF) and proteinuria (urine PCR 1.4 g/g) remained persistent even 1 week later; after 2 months, the condition of the patient still had not improved, thus prompting the use of steroid pulse therapy. Morisawa and Honda [15] reported a 16-year-old male with flare up of known IgAN after the second dose of Pfizer vaccination; gross hematuria occurred on the day of vaccination, and the authors initiated pulse therapy of methylprednisolone 1 g over 2 weeks from 35 days post vaccination followed by prednisolone 60 mg every other day. Since the patient showed an aggravated creatinine level of 1.29 mg/dL on day 55, prednisolone was used daily for 4 weeks; azathioprine was then added. In contrast, our patient recovered relatively rapidly because treatment was initiated earlier than in the above cases. The urine PCR (0.34 g/g) and microscopic hematuria decreased approximately 1 month after treatment. The urine PCR reduced more effectively to within normal range (0.19 g/g) 2 months later. Microscopic hematuria was resolved completely after 4 months. We administered oral steroid and ACE inhibitor to our patient from the onset of symptoms. According to the study by Klomjit et al. [6], the COVID-19 vaccine may stimulate preexisting IgAN by immune activation in susceptible patients rather than lead to the development of de novo IgAN. In patients with IgAN, 30% progressed to AKI and 40% had massive proteinuria after COVID-19 vaccination [5]. In the present case, our patient did not develop AKI. Her clinical manifestations of macroscopic hematuria and proteinuria improved earlier than in the abovementioned pediatric cases [5,15]. It was unclear whether IgA deposits were present in the kidney before COVID-19 vaccination or whether new IgA antibodies were deposited after vaccination. In either case, we should closely monitor the possibility of relapse and de novo IgAN after mRNA-based COVID-19 vaccination. Serial assessments of proteinuria and kidney function should also be performed for high-risk patients.
We report a rare case of newly diagnosed IgAN associated with mRNA-based COVID-19 vaccine in a 12-year-old girl who presented with gross hematuria and heavy proteinuria. While the short-term clinical outcome seems favorable, we need to establish long-term follow-up of our patient. Further studies with a larger scale of cases are required to unearth the precise mechanisms of IgAN following mRNA-based COVID-19 vaccination. Pediatricians should also take into account the possibility of renal complications after COVID-19 vaccination and during COVID-19 infection.
Notes
Ethical statements
This report was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB No. 2022AS0217). Informed consent was waived.
Conflicts of interest
Hyung Eun Yim is an editor-in-chief and Kee Hwan Yoo is an editorial board member of the journal. However, they were not involved in the peer reviewer selection, evaluation, or decision processes of this article. The authors have no other potential conflicts of interest relevant to this article to disclose.
Acknowledgments
We would like to express our gratitude to Professor Bo-Kyung Je (Department of Radiology, Korea University Ansan Hospital, Korea University College of Medicine) for performing the kidney biopsy.
Author contributions
Conceptualization: HEY, KHY
Data curation: DYK, HEY, MHS, KHY
Investigation: DYK
Methodology: DYK, HEY, MHS
Project administration: HEY
Visualization: DYK
Writing-original draft: DYK
Writing-review & editing: DYK, HEY, MHS, KHY
All authors have read and approved the final manuscript.
References
1. Cambier A, Boyer O, Deschenes G, Gleeson J, Couderc A, Hogan J, et al. Steroid therapy in children with IgA nephropathy. Pediatr Nephrol 2020;35:359-66.


2. Punj S, Eng E, Shetty AA. Coronavirus disease 2019 and kidney injury. Curr Opin Nephrol Hypertens 2021;30:444-9.


3. Park JS, Lee EY. Renal side effects of COVID-19 vaccines in patients with immunoglobulin A nephropathy. Kidney Res Clin Pract 2022;41:124-7.



4. Serafinelli J, Mastrangelo A, Morello W, Cerioni VF, Salim A, Nebuloni M, et al. Kidney involvement and histological findings in two pediatric COVID-19 patients. Pediatr Nephrol 2021;36:3789-93.



5. Horino T, Sawamura D, Inotani S, Ishihara M, Komori M, Ichii O. Newly diagnosed IgA nephropathy with gross haematuria following COVID-19 vaccination. QJM 2022;115:28-9.


6. Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep 2021;6:2969-78.



7. Paranhos RM, De Souza Figueiredo GA, De Abreu GR, Ferreira GC, Fonseca GG, Simoes E Silva AC. Immunoglobulin A nephropathy in paediatrics: an up-to-date. Nephrology (Carlton) 2022;27:307-17.


8. Bitencourt L, Pedrosa AL, de Brito SB, Froes AC, de Carvalho ST, Fonseca GG, et al. COVID-19 and renal diseases: an update. Curr Drug Targets 2021;22:52-67.


9. Wisnewski AV, Campillo Luna J, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One 2021;16:e024949.

10. Agrati C, Castilletti C, Goletti D, Meschi S, Sacchi A, Matusali G, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms 2021;9:1315.



11. Udagawa T, Motoyoshi Y. Macroscopic hematuria in two children with IgA nephropathy remission following Pfizer COVID-19 vaccination. Pediatr Nephrol 2022;37:1693-4.



12. Kudose S, Friedmann P, Albajrami O, D'Agati VD. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int 2021;100:468-9.



13. Abdel-Qader DH, Hazza Alkhatatbeh I, Hayajneh W, Annab H, Al Meslamani AZ, Elmusa RA. IgA nephropathy in a pediatric patient after receiving the first dose of Pfizer-BioNTech COVID-19 vaccine. Vaccine 2022;40:2528-30.



Fig.┬Ā1.
Microscopic findings from the kidney biopsy. (A) Mesangial cell proliferation (black arrows). (B) Interstitial fibrosis (black arrow). (C) Immunoglobulin A deposits. (D) Electron dense deposits (white arrows) in the mesangial area (A and B: hematoxylin and eosin stain, ├Ś40 and ├Ś20; C: immunofluorescence staining, ├Ś20; D: electron microscopy, ├Ś6,000).
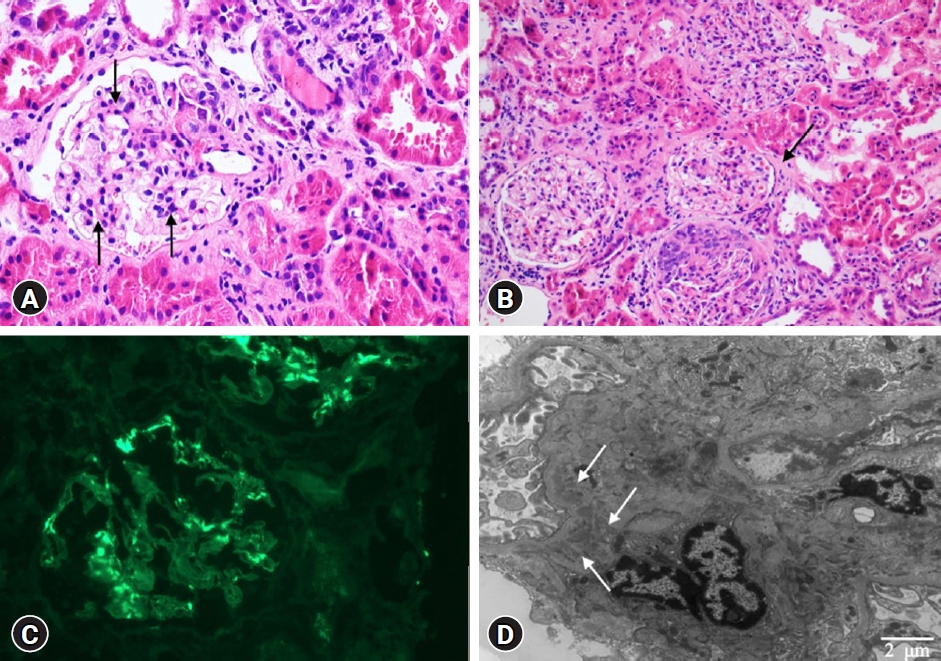
Fig.┬Ā2.
Clinical course of our patient. The quantity of urine RBCs is scored according to five stages (0: 1ŌĆō4 RBCs/HPF; 1: 5ŌĆō9 RBCs/HPF; 2: 10ŌĆō29 RBCs/HPF; 3: 30ŌĆō60 RBCs/HPF; 4: >60 RBCs/HPF). RBCs, red blood cells; HPF, high power field; Cr, creatinine; PCR, protein-to-creatinine ratio; mRNA, messenger RNA; COVID-19, coronavirus disease of 2019.
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 2,358 View
- 27 Download
- ORCID iDs
-
Do Young Kim

https://orcid.org/0000-0002-5643-0582Hyung Eun Yim

https://orcid.org/0000-0001-9805-9278Min Hwa Son

https://orcid.org/0000-0002-4185-1712Kee Hwan Yoo

https://orcid.org/0000-0001-6490-4293 - Related articles
-
Nutcracker syndrome combined with immunoglobulin A nephropathy: two case reports2023 December;27(2)




