| Child Kidney Dis > Volume 19(2); 2015 > Article |
|
Abstract
Purpose:
Burkholderia cepacia is an aerobic, glucoseŌĆōnon-fermenting, gram-negative bacillus that mainly affects immunocompromised and hospitalized patients. Burkholderia cepacia has high levels of resistance to many antimicrobial agents, and therapeutic options are limited. The authors sought to analyze the incidence, clinical manifestation, risk factors, antimicrobial sensitivity and outcomes of B. cepacia urinary tract infection (UTI) in pediatric patients.
Methods:
Pediatric patients with urine culture-proven B. cepacia UTI between January 2000 and December 2014 at Samsung Medical Center, a tertiary referral hospital in Seoul, Republic of Korea, were included in a retrospective analysis of medical records.
Results:
Over 14 years, 14 patients (male-to-female ratio of 1:1) were diagnosed with B. cepacia UTI. Of 14 patients with UTI, 11 patients were admitted to the intensive care unit, and a bladder catheter was present in 9 patients when urine culture was positive for B. cepacia. Patients had multiple predisposing factors for UTI, including double-J catheter insertion (14.2%), vesico-ureteral reflux (28. 6%), congenital heart disease (28.6%), or malignancy (21.4%). Burkholderia cepacia isolates were sensitive to piperacillin-tazobactam and sulfamethoxazole-trimethoprim, and resistant to amikacin and colistin. Treatment with parenteral or oral antimicrobial agents including piperacillin-tazobactam, ceftazidime, meropenem, and sulfamethoxazole-trimethoprim resulted in complete recovery from UTI.
Burkholderia cepacia is an aerobic, glucoseŌĆōnon-fermenting, gram-negative bacillus that mainly affects immunocompromised and hospitalized patients as well as those with chronic granulomatous disease and cystic fibrosis [1-3]. There have also been reports of B. cepacia causing endocarditis, infections of the central nervous system, and neonatal sepsis [2-4]. This organism is not normal human flora, and is usually found in hospital environments, such as in contaminated disinfectants, nebulizer solutions, medical devices, and on the skin of healthcare workers [3,5-7]. Recently, B. cepacia infections have increased because of increased use of broad-spectrum antimicrobial agents, longer duration of hospitalization and indwelling device-related infections [4,7,8]. This organism has high levels of resistance to many antimicrobial agents, and sulfamethoxazole-trimethoprim has been the drug of choice for treatment [1,4].
There have been rare reports of urinary tract infection (UTI) caused by B. cepacia. Hosts with predisposing factors, such as post renal transplant, vesico-ureteral reflux (VUR), neurogenic bladder, bladder irrigation, or use of contaminated medical devices, have been reported to be susceptible to B. cepacia UTI [9-11]. We sought to analyze the incidence, clinical manifestations, risk factors, antimicrobial sensitivity and outcomes of B. cepacia UTI in pediatric patients.
This retrospective study was conducted at Samsung Medical Center, a tertiary referral hospital in Seoul, Republic of Korea. Patients with urine culture-proven B. cepacia UTI between January 2000 and December 2014 were included in the retrospective analysis of medical records. Urinary tract infection was defined as a positive urine test plus at least one of the symptoms or signs of infection, including temperature > 38Ōäā, dysuria, or costovertebral angle tenderness. A positive urine test was defined as a urine culture with Ōēź 105 colony forming units (CFU)/mL of B. cepacia from a urine sample collected either via catheter (if during the catheterization period), or by voiding (if the age was more than 3 years) or intermittent catheterization (if the age was less than 3 years). Collected data included gender, age, primary disease, risk factors, antimicrobial sensitivity and outcomes. Antimicrobial susceptibility was determined via VITEK 2 (Bio-Merieux, Durham, NC, USA) according to Clinical and Laboratory Standards Institute guideline. Result with intermediate was considered as resistance.
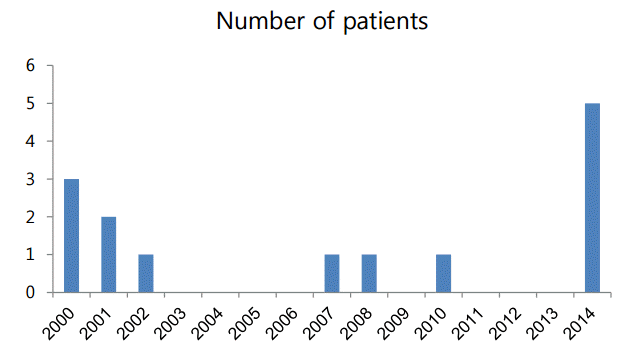
During 14 years, 14 patients (male-to-female ratio of 1:1) were diagnosed with B. cepacia UTI. The annual incidence of B. cepacia UTI is shown in Fig. 1. Although B. cepacia UTI sporadically occurred from 2000 to 2013, 5 such patients (35.7%) were treated in the pediatric intensive care unit in 2014.
The characteristics of the studied patients are presented in Table 1. Of 14 patients with UTI, 11 patients were admitted to the pediatric intensive care unit. Nine of these 11 patients had a bladder catheter (Foley) in place when urine culture grew B. cepacia. Patients had multiple predisposing factors for UTI, including double-J catheter insertion, VUR, congenital heart disease, or malignancy.
Two patients developed double-J catheter-related B. cepacia UTIs, and were treated with parenteral antimicrobial agents. Subsequent urine culture was negative after treatment, and they were discharged after the removal of the double-J catheter. During follow-up, they did not develop recurrent UTI.
Four patients with VUR developed B. cepacia UTI. A 1-month-old girl with a cloaca anomaly associated VUR developed UTI after colostomy operation. A subsequent urine culture was negative after the treatment, and she was discharged with no further antimicrobial agents. During follow-up, the patient did not develop recurrent UTI. A 2-month-old boy with bilateral VUR grade IV developed B. cepacia UTI in spite of chemoprophylaxis, and follow-up urine culture was negative after the treatment. He suffered from recurrent UTI, and ureteroneocystostomy was performed at the age of 18 months. A 2-year-old boy with Rubinstein-Taybe syndrome and bilateral VUR developed B. cepacia UTI after the operation of ureteroneocystostomy under the condition of PCN. After the treatment, he was discharged after removal of PCN, and did not suffer from recurrent UTI. Finally, a 1-month-old boy with ventricular septal defect (VSD) and a single kidney associated with VUR developed B. cepacia UTI despite the use of a third-generation cephalosporin, and levofloxacin was given intravenously for 14 days, and the infection resolved.
Four patients with congenital heart disease were diagnosed with B. cepacia UTI from bladder catheter urine samples. Three patients were admitted to the pediatric intensive care unit for congenital heart disease repair, and febrile UTI developed while a bladder catheter was in place after the operation. They were treated with intravenous antimicrobial, and the subsequent urine culture showed no growth. Finally, a 2-month-old boy with total anomalous pulmonary venous return was admitted to the pediatric intensive care unit because of severe respiratory distress. While in the intensive care unit receiving mechanical ventilation, he developed fever despite use of a third-generation cephalosporin. His urine sample from a bladder catheter grew over 105 CFU/mL of B. cepacia. The patient was treated with parenteral meropenem for 14 days. The subsequent urine culture was negative, but he died of uncompensated respiratory failure.
Three patients had malignancies including leukemia, glioblastoma, and neuroblastoma, and 2 patients with leukemia and neuroblastoma were on chemotherapy. A 12-year-old girl with acute lymphoblastic leukemia developed B. cepacia UTI during treatment with parenteral antimicrobial agents (cefotaxime and amikacin). She was treated with parenteral imipenem for 14 days, and follow-up urine culture did not grow any organisms. Unfortunately, she died of uncontrolled sepsis. Next, an 18-year-old girl with glioblastoma developed B. cepacia UTI after tumor removal, and treated with parenteral piperacillin-tazobactam for 14 days. The subsequent urine culture was negative. An 8-day-old girl with prenatally diagnosed neuroblastoma developed B. cepacia UTI after the first cycle of chemotherapy during the use of empirical antimicrobial agents (cefotaxime and amikacin). The antimicrobial agents were changed to parenteral meropenem, and urine culture demonstrated clearance after treatment. Finally, a 3-month-old boy who was born at a gestational age of 25+2 weeks developed B. cepacia UTI during the hospitalization of neonatal intensive care unit. He was treated with oral cefdinir, and follow-up urine culture was negative. He improved clinically and was discharged without the need for further antimicrobial agents.
Pediatric patients were treated with piperacillin-tazobactam, ceftazidime, meropenem, levofloxacin, sulfamethoxazole-trimethoprim, and other third-generation cephalosporins for 7 to 14 days. Most patients were treated with parenteral antimicrobial agents. Follow-up urine cultures were sterile in all patients after this treatment period. The antimicrobial sensitivity pattern of B. cepacia is shown in Figure 2. B. cepacia isolates were sensitive to piperacillintazobactam and sulfamethoxazole-trimethoprim, and resistant to amikacin and colistin.
Burkholderia cepacia usually causes nosocomial infections in immunocompromised hosts, and the most common infectious focus is the respiratory tract, followed by intravascular catheters [2,3,8]. Burkholderia cepacia survives in moist environments, and outbreaks of B. cepacia infection have been described in association with contaminated nebulizers, indigo-carmine dye, mouthwash, and moisturizing body milk [3,5-7]. In our study, the incidence of B. cepacia in 2014 was relatively high, and surveillance cultures for intensive care unit environments were conducted; however, negative results were found.
There have been few reports of the characteristics of B. cepacia UTI. Affected patients often have predisposing factors, such as renal transplantation, prolonged bladder catheterization, or urethrocystoscopy [9-11]. In our study, predisposing host factors such as prolonged genitourinary catheterization, VUR, congenital heart disease, and immunocompromised status were suggested. Twelve of 14 patients with B. cepacia UTI had genitourinary catheterization such as bladder catheter, PCN, or double-J stents. Zeeshan et al. reported that VUR in a renal transplant recipient was a risk factor for B. cepacia UTI [11]. In our study, 4 patients (29%) showed VUR-related UTI in spite of prophylactic antimicrobial agents. VUR was also associated with other anomalies such as cloaca anomaly or chromosome abnormality. In cases of congenital heart disease, patients required prolonged pediatric intensive care unit stays and bladder catheterization, which increased their susceptibility to B. cepacia UTI. In addition, immunocompromised oncology patients have been reported to be susceptible to B. cepacia infection [8].
Burkholderia cepacia is a multidrug-resistant organism, and therapeutic options are limited [1]. Although trimethoprim-sulfamethoxazole has been the drug of choice, it is difficult to administer because of hypersensitivity, lack of availability, and resistance in some cases. Avgeri et al. reported that ceftazidime, meropenem, and piperacillin, either alone or in combination, may be used as alternative options in B. cepacia infections [1]. Patra et al. reported that piperacillin-tazobactam, ciprofloxacin, and trimethoprim-sulfamethoxazole, either alone or in combination, could result in complete recovery of B. cepacia sepsis in neonates. The highest susceptibility was observed with meropenem [4]. In our study, piperacillin-tazobactam, ceftazidime, trimethoprim-sulfamethoxazole, levofloxacin, and meropenem were used in the majority of cases. All patients experienced complete recovery from UTI. In our study, the highest susceptibility was observed with piperacillin-tazobactam and trimethoprim-sulfamethoxazole. Importantly, there was 100% resistance to amikacin and colistin. Even so, Li et al. reported a case of B. cepacia UTI after renal transplantation that required a graft nephrectomy because B. cepacia showed in vivo resistance to all available antimicrobial agents, and long-term use of piperacillin could not resolve the septic foci [9]. Because of such antimicrobial resistance, a combination of antimicrobial agents and surgical treatment in some cases may be required.
Burkholderia cepacia is a pathogen with intrinsic resistance to numerous antimicrobial agents that causes nosocomial UTI in pediatric patients with risk factors such as prolonged genitourinary catheterization, VUR, congenital heart disease, or malignancy. Prompt removal of catheters and appropriate antimicrobial therapy for B. cepacia UTI in high-risk patients can ensure complete recovery. In addition, a surveillance program for nosocomial infection in intensive care units is necessary to prevent B. cepacia infections.
References
1. Avgeri SG, Matthaiou DK, Dimopoulos G, Grammatikos AP, Falagas ME. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int J Antimicrob Agents 2009;33:394-404.


2. Lu DC, Chang SC, Chen YC, Luh KT, Lee CY, Hsieh WC. Burkholderia cepacia bacteremia: a retrospective analysis of 70 episodes. J Formos Med Assoc 1997;96:972-8.

3. Pegues CF, Pegues DA, Ford DS, Hibberd PL, Carson LA, Raine CM, et al. Burkholderia cepacia respiratory tract acquisition: epidemiology and molecular characterization of a large nosocomial outbreak. Epidemiol Infect 1996;116:309-17.



4. Patra S, Bhat YR, Lewis LE, Purakayastha J, Sivaramaraju VV, Kalwaje EV, et al. Burkholderia cepacia sepsis among neonates. Indian J Pediatr 2014;81:1233-6.


5. Alvarez-Lerma F, Maull E, Terradas R, Segura C, Planells I, Coll P, et al. Moisturizing body milk as a reservoir of Burkholderia cepacia: outbreak of nosocomial infection in a multidisciplinary intensive care unit. Crit Care 2008;12:R10.

6. Gravel D, Sample ML, Ramotar K, Toye B, Oxley C, Garber G. Outbreak of burkholderia cepacia in the adult intensive care unit traced to contaminated indigo-carmine dye. Infect Control Hosp Epidemiol 2002;23:103-6.

7. Martin M, Winterfeld I, Kramme E, Ewert I, Sedemund-Adib B, Mattner F. Outbreak of Burkholderia cepacia complex caused by contaminated alcohol-free Mouthwash. Anaesthesist 2012;61:25-9.


8. Durham SH, Lee AE, Assanasen C. Burkholderia cepacia septicemia in a pediatric oncology patient: a pharmacotherapy challenge. Ann Pharmacother 2012;46:e16.

9. Li FK, Chan KW, Chan TM, Lai KN. Burkholderia urinary tract infection after renal transplantation. Transpl Infect Dis 2003;5:59-61.


Fig.┬Ā2.
Antimicrobial susceptibility of B. cepacia isolates Abbreviations: Pip-tazo, Piperacilline-tazobactam; SXT, trimethoprim-sulfamethoxazole.

Table┬Ā1.
Demographic characteristics and risk factors of 14 pediatric patients with Burkholderia cepacia urinary tract infection
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :
-
METRICS

-
- 3 Crossref
- 0 Scopus
- 14,024 View
- 140 Download
- Related articles
-
Pneumocystis Pneumonia after Kidney Transplantation in Children2020 April;24(1)
Treatment for Urinary Tract Infection of Children in Korea2001 April;5(1)