Enuresis as a Presenting Symptom of Graves’ Disease: A Case Report
Article information
Abstract
Enuresis is intermittent urinary incontinence during sleep at night in children aged 5 years or older. The main pathophysiology of enuresis involves nocturnal polyuria, abnormal sleep arousal, and low functional bladder capacity. In rare cases, enuresis is an early symptom of endocrine disorders such as diabetes or thyroid disorders. Herein, we report a case of a 12-year-old girl with enuresis as a rare initial presentation of Graves’ disease. She complained of nocturnal enuresis from a month before visiting our clinic. She also complained of urinary frequency, headache, and weight loss. On physical examination, she had tachycardia, intention tremors, and a diffuse goiter on her anterior neck with bruit on auscultation. Her thyroid function test results revealed hyperthyroidism, and Graves’ disease was diagnosed as the thyroid stimulating hormone receptor autoantibody was positive. After treatment for Graves’ disease with methimazole, symptoms of enuresis resolved within 2 weeks as she became clinically and biochemically euthyroid. In children with secondary enuresis, Graves’ disease should be considered as a differential diagnosis, and signs of hyperthyroidism should be checked for carefully.
Introduction
Enuresis is common in children. About 15–20% of 5-year-olds suffer from nocturnal enuresis. Of these, 15% experience spontaneous remission each year, and the prevalence of enuresis in teens is about 3%. Children who have never been dry at night are classified as those with “primary enuresis” and children who have previously been dry at night for at least 6 months are classified as those with “secondary enuresis.” Although the clinical presentation of children with primary or secondary enuresis is similar, children with secondary enuresis are more likely to have conditions that may precipitate enuresis than children with primary enuresis [1]. Several conditions may coexist with enuresis. Diabetes mellitus and diabetes insipidus may lead to polyuria [2]. Sleep-disordered breathing impairs arousal from sleep. Conditions such as cystitis, constipation, urethral obstruction, and neurogenic bladder reduce functional bladder capacity which can cause enuresis [3,4]. Detecting comorbid conditions in children with enuresis is important because it affects the treatment response and ultimate prognosis.
Graves’ disease is an autoimmune disease characterized by autoantibodies toward the thyroid stimulating hormone (TSH) receptors. Graves’ disease is the most common cause of hyperthyroidism with suppressed serum TSH and elevated free thyroxine (T4) and triiodothyronine (T3) levels. Its common clinical presentations are weight loss, palpitations, tremors, anxiety, diarrhea, and heat intolerance. Physical findings of Graves’ disease include a diffusely enlarged thyroid, tachycardia, exophthalmos, and lid retraction. Although nocturnal enuresis is not a classical feature of Graves' disease, there are some reports of enuresis in Grave's disease.
Herein, we report a case of a 12-year-old girl with secondary enuresis as an initial presentation of Graves’ disease. Her symptoms recovered after achieving euthyroidism.
Case report
A 12-year-old girl visited our pediatric nephrology outpatient clinic for enuresis. She had been dry at night after the age of 4 years, but complained of enuresis for 6 times a week from a month ago. She also complained of a high daytime voiding frequency and a headache that started 2 years prior and was aggravated 7 months ago. She also had alopecia totalis from 12 months of age.
She was 165 cm tall (95–97th percentile) and weighed 44.5 kg (25–50th percentile). She had lost approximately 5 kg of weight over the past year. She did not have the symptoms of vomiting, diarrhea, or constipation. Her initial vital signs were as follows: blood pressure, 110/80 mmHg; heart rate, 100 beats/min; respiratory rate, 20 breaths/min; and body temperature 37.1℃. On physical examination, she had a diffuse swelling on the anterior neck that was compatible with goiter grade II according to World Health Organization (WHO) grading (Fig. 1) [5]. A bruit was auscultated at the goiter, and she had an intention tremor. Over 18 hours, her urine output was 1,450 mL, which indicated that she did not have polyuria.
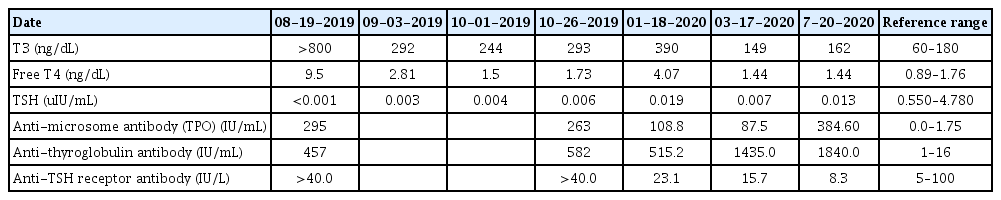
Her urine specific gravity was normal (S.G. 1.027), and there was no proteinuria, hematuria, or pyuria. Her simple abdomen x-ray image shown nonspecific bowel gas patterns without evidence of fecal impaction. The results of her initial blood tests were normal for complete blood count (white blood cells 7,860/μL, hemoglobin 12.8 g/dL, platelets 414,000/μL), liver and renal functions (aspartate aminoaminotransferase 36 IU/L, alanine aminotransferase 53 IU/L, blood urea nitrogen 14.9 mg/dL, creatinine 0.25 mg/dL), and acid-base balance and electrolytes (pH 7.442, PCO2 30 mmHg, sodium 138 mmol/L, potassium 3.9 mmol/L, chloride 104 mmol/L, bicarbonate 23.6 mmol/L). Thyroid function tests were performed as the patient had headaches, tachycardia, and a goiter on physical examination. Thyroid function test results revealed hyperthyroidism with a decreased TSH level of <0.001 uIU/mL (reference range 0.51– 4.94 uIU/mL), elevated free T4 of 9.5 ng/dL (reference range 0.89–1.76 ng/dL), and T3 of >800 ng/dL (reference range 60–181 ng/dL). Graves’ disease was diagnosed because the anti-TSH receptor antibody level was over 40.0 IU/L (reference range 0–2.0 IU/L) (Table 1). Her thyroid ultrasonography results revealed both thyroid glands to be markedly enlarged with heterogeneous parenchymal echogenicity, which were findings all highly suggestive of diffuse thyroid disease (Fig. 2).

Ultrasonography images of the enlarged thyroid gland. Isthmus (A), left lobe (B), and right lobe (C) of the thyroid gland.
Treatment was initiated with methimazole and propranolol hydrochloride. Her symptoms, including nocturnal enuresis, improved within 2 weeks of medication. Methimazole dosages were gradually reduced while propranolol was stopped 2 weeks after initial administration. Currently, she is being followed up at the outpatient clinic with improved thyroid function test results (Table 1). Her laboratory tests were nearly euthyroid (TSH 0.013 ng/dL, free T4 1.44 ng/dL, T3 162 uIU/mL) on her last visit without showing any adverse events due to methimazole; no drug eruption, no signs of leukopenia, and no liver function test abnormalities (Table 2). She no longer had symptoms, including nocturnal enuresis.
Discussion
We described a case of a 12-year-old girl with secondary enuresis and daytime urinary frequency. Her enuresis began recently, and she did not complain of any symptoms including increased thirst, voiding difficulties, encopresis, snoring, fatigue, or behavioral problems suggesting diabetes, constipation, sleep disorders, or neuropsychological disorders. Alternatively, she had headaches, hand tremors, weight loss, and a palpable goiter on her anterior neck. Thyroid disease was strongly suspected, and she was eventually diagnosed with Graves’ disease based on the thyroid function tests results. Considering that enuresis improved within 2 weeks of starting medications for Graves’ disease as she became clinically and biochemically euthyroid, it is thought that her thyroid dysfunction precipitated secondary enuresis.
There are two previous reports of children with hyperthyroidism presenting with nocturnal enuresis as the primary symptom. Andrea et al. diagnosed Graves’ disease in 6-year-old twins presenting with urinary frequency and nocturnal enuresis as the primary symptoms [6]. Meir et al. diagnosed Graves’ disease in a 9-year-old boy who presented with bedwetting after staying dry since he was 5 years old7). These patients also presented with sinus tachycardia and a goiter, as in our case. To the best of our knowledge, we have reported the third case of diagnosing Graves’ disease in a child who visited with nocturnal enuresis as a chief complaint.
The exact mechanism of intermittent incontinence during sleep in patients with hyperthyroidism remains uncertain. However, there are three possible explanations for this phenomenon. One is that cellular response to adrenergic activity can be increased by thyroid hormone. Two is that catecholamine levels can be increased by thyroid hormone. These may cause an activation of the sympathetic nervous system [8]. The typical clinical manifestations of hyperthyroidism, such as weight loss, tachycardia, and sweating, are caused by an autonomic nervous system imbalance and sympathetic overactivity. A balance between the sympathetic and parasympathetic nervous systems is crucial for normal bladder function. Elevation of beta-adrenergic activity can result in enuresis and, accordingly, thyrotoxicosis can result in bladder control and micturition symptoms [9]. The third explanation is that a hyperthyroid state increases glomerular filtration and water intake, leading to nocturnal polyuria [10]. Results of an animal study by Wang et al. showed a downregulation of aquaporin water channels and an increase in solute excretion in hyperthyroid rats [11]. These changes can enhance polyuria and lead to enuresis.
This is a rare case of enuresis caused by hyperthyroidism. Based on this case, pediatricians should be made aware that hyperthyroidism could precipitate nocturnal enuresis in children and adolescents. These patients should be checked carefully for symptoms and signs of hyperthyroidism. A thyroid function test is recommended when hyperthyroidism is suspected.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Patient consent
This study was approved by the institutional review board of Hallym University Kangnam Sacred Heart Hospital, and the consent was waived due to the nature of the retrospective study (IRB number 2020-08-002-002).