Introduction
Antenatal urinary tract dilatation (UTD) is commonly found on routine fetal ultrasound [1,2]. UTD is a preferred term while various terminology such as congenital, fetal, antenatal, or prenatal hydronephrosis has been used for years [3,4]. Given that it is associated with a broad spectrum of conditions ranging from a transient finding to congenital abnormalities of the kidney and urinary tract (CAKUT) leading to chronic kidney disease (CKD), it is important to avoid unnecessary testing and identify cases of significant urinary tract anomaly. Nguyen et al. [4] have recently updated the UTD classification system. A new guideline was subsequently developed for the evaluation of antenatal and postnatal UTD [4]. This review aimed to discuss a clinically significant question evaluating and managing antenatal UTD from a pediatric nephrologistŌĆÖs view and to assist pediatricians in their decision-making about the antenatal UTD.
Q1. What is the definition of UTD?
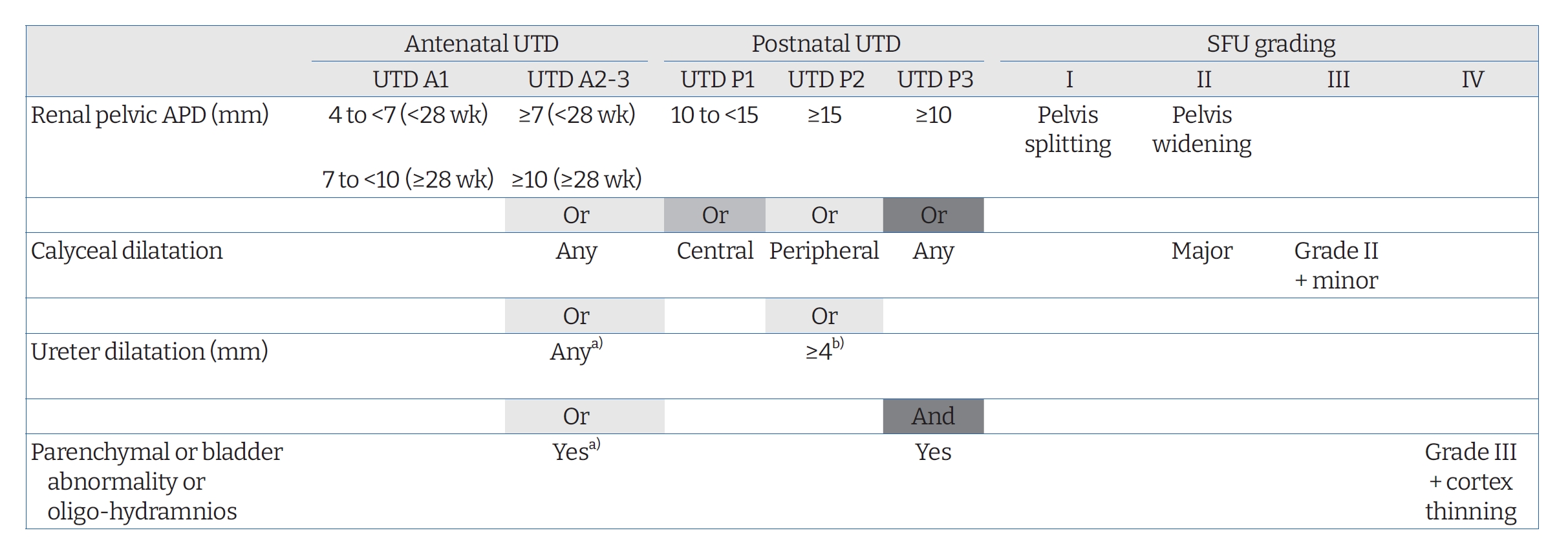
Different grading systems for UTD have been developed for a long time. The Society for Fetal Urology (SFU) system and renal pelvis anterior-posterior diameter (APD) measurements for grading UTD are commonly used [5]. However, the recently updated UTD classification [4] seems to be appropriate for predicting long-term renal outcome and need for surgery in addition to identifying postnatal CAKUT [6-8]. According to the UTD classification system [4], antenatal UTD is defined as a renal pelvis APD of Ōēź4 mm in the second trimester (<28 weeks) and/or Ōēź7 mm in the third trimester (Ōēź28 weeks). The new system focuses not only on the kidney, but also on the entire urinary tract system, classifying UTD into two antenatal categories (UTD A1, UTD A2-3) and three postnatal categories (UTD P1, P2, and P3). UTD A1 is considered at low risk for postnatal CAKUT based on APD of 4 to <7 mm at <28 weeks and APD of 7 to <10 mm at Ōēź28 weeks. Abnormal kidney parenchyma (cortical thinning, hyperechogenicity, cystic dysplasia, or indistinct corticomedullary differentiation), calyces, ureters, bladder (wall thickening, ureterocele, or dilated posterior urethra), or amniotic fluid accompanied with a renal APD of Ōēź7 mm at <28 weeks or Ōēź10 mm at Ōēź28 weeks corresponds to antenatal UTD A2-3, which is considered to be at an increased risk for postnatal CAKUT. At least 48 hours after birth, the presence of a renal pelvis APD of < 10 mm without other abnormalities (no calyceal or ureteral dilation, no abnormalities of renal parenchyma or bladder) is defined as normal in the UTD classification system. In this system, an APD of 10 to 15 mm or central calyx dilatation is defined as UTD P1 (low risk) and an APD of Ōēź15 mm or peripheral calyceal dilatation or dilated ureter of >4 mm with an APD of Ōēź10 mm or calyceal dilatation is defined as UTD P2 (intermediate risk). The presence of renal parenchymal abnormality, bladder abnormality, or oligohydramnios combined with an APD of Ōēź10 mm or any calyceal dilatation is classified as UTD P3 (high risk) [3,4]. UTD P1, UTD P2, and UTD P3 are comparable to SFU grade I-II, SFU grade III, and SFU grade IV, respectively (Fig. 1) [9].
Q2. What causes UTD?
The main etiologies of antenatally diagnosed antenatal UTD can be grouped into three broad categories: (1) physiologic or transient dilation; (2) vesicoureteral reflux (VUR); and (3) obstructive uropathy. As the degree of antenatal and postnatal UTD increases, there is an increased risk of CAKUT except VUR [10]. A recent paper reported that one-third of children with antenatal UTD had the UTD before birth and that the UTD was resolved or stabilized by the end of 2 to 3 years for another third of children while UTD persisted or CAKUT was diagnosed for the remaining cases [3]. Transient or physiologic UTD might be associated with hydration status, bladder filling, transient narrowing of the ureteropelvic junction (UPJ), or delayed maturation of ureteral peristalsis [11]. UPJ obstruction and VUR are the two most common CAKUT conditions, both of which can be diagnosed in 10% to 12% of cases [1,12]. Other CAKUT conditions causing UTD include ureterovesical junction obstruction, primary non-refluxing megaureter, bladder outlet obstruction including posterior urethral valve (PUV) or ureterocele, duplex collecting system, multicystic dysplastic kidney, and so on [3,11].
Q3. What is the optimal evaluation of UTD?
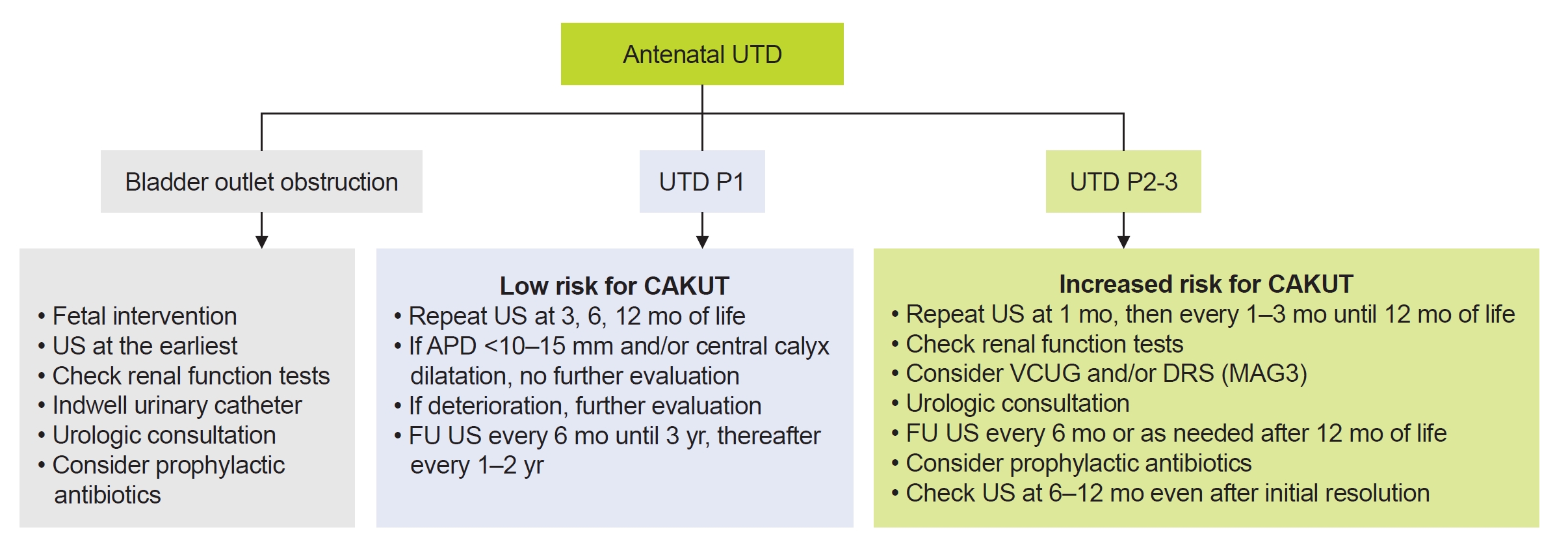
Extensive investigation for UTD is being proposed for cases with moderate or severe dilatation (UTD A2-3, P2, and P3). Nonetheless, it is recommended that all antenatal UTDs (UTD A1, A2-3) should be validated by two serial postnatal ultrasonography (US) at >48 hours after birth and a few weeks or months later [3,13]. For cases with suspected bladder outlet obstruction, kidney and bladder US should be checked as soon as possible postnatally and renal function should be checked together with indwelling urinary catheter. With urologic consultation, a follow-up US should be performed sooner [3,13]. For UTD P1, a follow-up US is recommended at 3, 6, and 12 months of life [3]. If there are only renal pelvis APD of <10 to 15 mm and/or central calyceal dilatation (UTD P1), further evaluation is not recommended [3]. However, peripheral calyceal dilation has been reported to increase the risk of a diagnosis of CAKUT [3,4]. If there are worsening findings on serial postnatal US, further work-up including renal function tests, voiding cystourethrogram (VCUG) or mercaptoacetyltriglycine (MAG3) scintigraphy (or diuretic renal scan) and urologic consultation should be considered. For UTD P2-3, a follow-up US in 1 to 3 months is suggested. Evaluation with renal function tests (especially serum creatinine, electrolytes, and blood gas analysis), VCUG, and MAG3 scan are considered [3,14]. Diuretic renal scan is usually performed from 6 to 8 weeks of age [3]. Measurement of serum cystatin C instead of creatinine may offer significant advantages in neonates and young infants given that serum cystatin C levels are less affected by age, sex, dietary protein intake, and muscle mass compared to creatinine [15]. Consultation to a pediatric urologist and the use of prophylactic antibiotics can be considered based on the severity of clinical conditions. Supplementary comments for the antibiotic prophylaxis will be mentioned in the following subject (Q5). A follow-up US at 6ŌĆō12 months even after initial resolution could be recommended for some cases with UTD P2-3 since a recurrence of significant UTD has been reported in patients with spontaneous improvement (Fig. 2) [14,16].
Q4. Who will need a urologic intervention?
The exact indications and suitable time for surgical intervention remain controversial. About 50% of postnatal UTDs will resolve and the remaining 40% to 45% will show improvement or stabilization of UTD within the first 3 years of life [3,14,17]. General indications for surgery include bladder outlet obstruction with oligohydramnios and recurrent urinary tract infections (UTIs) with VUR or UPJ obstruction. Increasing dilatation and/or decreasing split function (<40% with impaired renal drainage or >10% of renal function deterioration on a follow-up renal scan) are also indicative of the need for surgery [3,13,14]. The cumulative incidence of needing surgery was about 20% to 30% children with antenatal UTD in long-term studies [17-19], while another study reported a far smaller proportion of patients undergoing any surgical procedure [20]. Yang et al. [18] have found that the ipsilateral differential renal function is preserved only in the early pyeloplasty group, implying that early surgical treatment is important to preserve renal function in patients with persistent UTD P2-3 (SFU grade III or IV). In addition, several predictors for surgical intervention have been suggested, including initial postnatal APD, renal pyramidal thickness, delayed cortical tissue transit time on diuretic renal scan, and renal parenchyma-to-hydronephrosis area ratio [21-24].
Q5. What is the risk of UTI? Do we need an antibiotic prophylaxis?
In general, children with antenatally diagnosed UTD are at an increased risk of UTI. Infants with antenatal UTD are more likely to have acute pyelonephritis within the first year of life than those without UTD [25]. Several studies have shown a cumulative incidence of 7% to 14% for UTI during infancy [26-28]. While some studies reported higher incidence of 14% to 40% for UTI especially in cases with moderate or severe UTD [29,30], others revealed lower occurrence of UTI (3.3% to 6.83%) in children with antenatal diagnosis of UTD [31,32]. Among underlying uropathies, VUR has been shown to be the most important risk factor for UTI within the UTD population [28]. UTI rates were 3- to 6-fold higher in patients with hydroureteronephrosis than in those with isolated hydronephrosis according to a systematic review by Braga et al. [33]. For a long time, the use of continuous antibiotic prophylaxis (CAP) has been a challenging issue. A systematic review [34] has shown that uncircumcised boys and children with ureteral dilatation and/or high-grade UTD are more prone to develop UTI and that CAP is recommended for these subgroups of patients. However, benefits of CAP are limited in infants with mild to moderate UTD since the protective effect of CAP against UTI has not been revealed yet [34,35]. The use of CAP for UTI prevention in infants with prenatal UTD has been acknowledged as a low level of evidence by the American Urological Association, the SFU, and the Canadian Urological Association [33].
Q6. What is the long-term outcome of antenatally detected UTD?
While permanent kidney damage is known to occur in about 40% of children with moderate or severe UTD [3], only a few studies have provided long-term outcomes of antenatally detected UTD [18,20,36,37]. Costa et al. [19] have reported the development of a composite event of hypertension, proteinuria, and/or reduced estimated glomerular filtration rate (eGFR) in 5% of a cohort of 447 children with isolated antenatal APD Ōēź5 mm at a median follow-up of 6.4 years. However, children with mild UTD did not have any chronic kidney damage during the follow-up period. Another study by Herthelius et al. [20] has shown that none of the children with antenatally detected UTD has proteinuria or reduced eGFR during 12 to 15 years of follow-up. Among confirmed cases with postnatal renal APD >7 mm and/or kidney parenchyma, calyces, ureters, or bladder pathology, persistent UTD occurred in 15% and persistent kidney damage assessed by renal DMSA (technetium 99m dimercaptosuccinic acid) scan or US was developed in 32% to 39%. They have concluded that it is unnecessary to perform long-term follow-up or use CAP in children with postnatal APD Ōēż7 mm and normal renal parenchyma, calyces, ureters, and bladder. According to a recent report by Herthelius [3], CAKUT is less likely to be diagnosed afterward in children older than 1 year who have a renal APD <15 mm without other abnormal findings on repeated exams. In contrast, there is a different story for a much longer follow-up of children with CAKUT [38,39]. In a study performed by Sanna-Cherchi et al. [38], renal deterioration was not evident until late adolescence apart from PUVs and bilateral hypodysplasia. However, 58 (18.6%) of 312 patients with CAKUT had started dialysis by 30 years of age. Patients with single kidney and those with renal hypodysplasia combined with PUVs were at increased risks for dialysis (hazard ratios: 2.43 and 5.1, respectively) compared to those with renal hypodysplasia, multicystic kidney, or horseshoe kidney [38]. Another study has also revealed that end-stage kidney disease caused by CAKUT is developed more often in adult age than in pediatric age [39]. Using data on the incidence and prevalence of renal replacement therapy (RRT) in a total of 212,930 patients, the median age at RRT start was found to be 31 years for patients with CAKUT and 61 years for those with non-CAKUT [39]. Patients with renal dysplasia required RRT at a very young age (median, 16 years) compared with those in other CAKUT categories. The incidence of RRT due to reflux-associated pyelonephritis increased sharply during the first two decades, reaching its peak in the early 20s. However, 50% of patients with CAKUT did not start RRT before turning 40s [39]. These studies suggest that ongoing loss of remnant nephrons can lead to CKD progression across the entire age range. Mild forms of CAKUT, including low nephron endowment at birth, seem to be more frequent than expected and be revealed in later adulthood [40]. Effective transition strategies from pediatric to adult nephrology service would be essential to achieve disease-specific good-quality care for this group of patients [39]. Individualized follow-up and management plans for children with UTD and/or CAKUT should be applied based on recommendations from a pediatric nephrologist or urologist, or both.
Q7. What do we research for the optimal management of UTD?
Since 2014, many studies have been performed to validate the correlation between the UTD classification system and clinical outcomes [6-9,20]. For predicting various outcomes such as surgical intervention, UTI risk, and chronic kidney damage, further extensive evaluation regarding the grading system would be necessary to assess its utility. Meanwhile, over the years, numerous urinary and serum biomarkers for UPJ obstruction and VUR have been studied, including neutrophil gelatinase-associated lipocalin [41-43], cystatin C [41], kidney injury molecule-1 [44], monocyte chemoattractant protein-1 [44], ╬▓2-microglobulin [43], and so on. Further studies are also needed to confirm the efficacy of these biomarkers to predict the development and progression of CKD as well as CAKUT itself. In addition, in line with the artificial intelligence era, investigations for grading UTD using machine learning algorithms (automated convolutional neural network model) have been reported. It was reported that a machine learning model classified 94% to 97.6% of patients correctly or within one grade of the diagnosis of radiologists of UTD [45,46]. Deep learning model could also predict renal complications in children with antenatal UTD concerning UPJ obstruction [47]. These models may offer great promise in their ability to affect clinical decision-making with a large amount of supplemental analytical data. Since genetic and environmental contributions for CAKUT have been identified, long-term prospective studies of patients with CAKUT coupled with comprehensive genomic analysis, functional validation of genetic variants, and an in-depth assessment of the in utero or perinatal environment are needed [40,48]. Recent advances in genetics, epigenetics, and molecular medicine might also offer an opportunity to expand our knowledge on the development of CAKUT and the proper management of patients with this condition.
Conclusion
Optimal evaluation of antenatal and/or postnatal UTD is essential as children with clinically significant abnormalities need to be identified while avoiding unnecessary testing. While most children with antenatal UTD have a favorable long-term outcome with a low risk of kidney disease progression, a greater portion of children with CAKUT need RRT during adulthood than during childhood. There is no definite answer to the question of at what time point we can stop the follow-up safely in a child with persistent UTD. In children with persistent moderate or severe UTD (UTD P2-P3, SFU III-IV), a non-negligible risk of permanent kidney damage exists. To improve the evaluation and management of these patients, future research studies should perform additional risk stratification and develop evidence-based interventions.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print