| Child Kidney Dis > Volume 19(2); 2015 > Article |
|
Abstract
Background:
We conducted this experimental study to examine whether human adipose-derived stem cells (ADSCs) are effective in achieving a recovery of damaged renal tubular epithelial cells in an animal model of cisplatin-induced acute kidney injury using rats.
Methods:
To examine the in vitro effects of ADSCs in improving nephrotoxicity, we treated mouse renal tubular epithelial cells with both ADSCs and cisplatin mouse renal tubular epithelial cells. And we equally divided 30 male white Sprague-Dawley (SD) rats into the three groups: the control group (intraperitoneal injection of a sterile saline), the cisplatin group (intraperitoneal injection of cisplatin) and the ADSC group (intraperitoneal injection of cisplatin and the hADSC via the caudal vein). At five days after the treatment with cisplatin, serum levels of blood urine nitrogen (BUN) and creatinine were measured from each SD rat. We performed histopathologic examinations of tissue samples obtained from the kidney.
Results:
The degree of the expression of TNF-╬▒ and that of Bcl-2 were significantly higher and lower respectively, in cisplatin group (P<0.05). Serum levels of BUN (P=0.027) and creatinine (P=0.02) were significantly higher in cisplatin group. On histopathologic examinations, there was a significant difference in the ratio of the renal injury between cisplatin group and ADSC group (P=0.002).
Acute kidney injury (AKI) is characterized by an abrupt impairment of the major functions of the kidney, such as the excretion of waste products, urine concentration and water and electrolyte balance. Its relatively higher prevalence has been well documented in the literature. In addition, its mortality in intensive-care-unit (ICU) patients is so high as to reach up to 50-80% [1]. Furthermore, its major etiologies include nephrotoxicity or ischemic renal injury. Finally, it is histopathologically characterized by acute tubular necrosis [2].
In patients with AKI, it would be mandatory not only to make a recovery of the renal function but also to regenerate the renal tubular epithelial cells [2]. To date, symptomatic treatments have been considered a mainstream modality in these patients, for which clinicians use appropriate fluid therapy, diuretics and control the acidity or electrolyte balance. Moreover, clinicians may also wait until patients achieve a recovery of the renal functions during the dialysis [3-5]. Although considerable efforts have been made to minimize the mortality up to present, there are no improvements in the prognosis of patients with AKI [6].
It is possible to create an experimental model of AKI by either ligating the renal vessels or administering pharmacological agents to experimental animals. The former is advantageous in simulating a clinical setting and reducing the degree of renal perfusion. It is disadvantageous, however, in that it may poorly simulate a clinical setting because there is a notable variability in the degree of the epithelial necrosis depending on the period of ligation. The latter is therefore performed to resolve this.
Cisplatin is one of the most popular anti-cancer drugs, and it has recently been used to create an animal model of AKI [2,7]. Its efficacy against malignancies, such as testis cancer, small cell lung cancer, head-and-neck cancer and bladder cancer, has been well documented [8,9]. But its adverse effects, such as ischemic or toxic injuries to the kidney leading to AKI arising from a loss of tubular epithelial cells, have also been reported [2].
Stem cells are such pluripotential ones as to self-replicate and to differentiate into other types of cells. Mesenchymal stem cells can be isolated from bone marrow, periosteum, synovium, skeletal muscle or adipose tissue [10]. Recent studies have shown that bone marrow-derived stem cells (BMSCs) play a key role in the regeneration of the necrotized renal tubules [2,11,12]. As compared with BMSCs, however, it is easier to harvest a greater amount of adipose-derived stem cells (ADSCs) at a time [13]. Similarly to BMSCs, they can also differentiate into other types of cells such as osteocytes, adipocytes, myocytes or chondrocytes [14].
Human ADSCs are byproducts of liposuction and they are abundantly present in patients with abdominal obesity. Therefore, they are of great use for stem cell studies [15].
To date, several authors have conducted stem cell studies to examine whether they are effective in improving the AKI. In 2007, Bi et al. created an animal model of cisplatin-induced AKI using rats and performed an intraabdominal injection of BMSCs or ADSCs. Then, these authors compared the viability of renal tubular cells, serum blood urea nitrogen (BUN) levels and histopathologic findings between the two groups, thus reporting that the degree of treatment effect was significantly higher in the rats receiving stem cell transplant [16]. It has also been reported that the stem cells were effective in regenerating the damaged renal tissue in an animal experimental model of cisplatininduced AKI [2,7].
Given the above background, we conducted this experimental study to examine whether human ADSCs have a beneficial effect in achieving a recovery of damaged renal tubular epithelial cells in an animal model of cisplatininduced AKI using rats.
We obtained ADSCs from liposuction in patients with abdominal obesity at department of plastic and reconstructive surgery. The adipose tissue was rinsed with cool phosphate buffered saline (PBS) (Gibco-BRL, Annapolis, MD, USA) three times in a clean bench. Then, it was sectioned using scissors or surgical blade and rinsed with PBS containing 1% antibiotics-antimicotics (Gibco-BRL) twice. After the dissolution of type II collagenase (Worthington Biochemical, Lakewood, NJ, USA) in DulbeccoŌĆÖs Modified Eagle Medium (DMEM) (Gibco-BRL) at a total concentration of 0.2%, we filtered the adipose tissue adipose tissue sections through a 0.2-mm membrane. They were mixed with the filtrate at a ratio of 1:2. Thus, the sample was prepared and then centrifuged twice at 100 rpm in a shaking incubator heated at 37┬░C for 30 minutes. The ADSCs were harvested, suspended and filtered through a 70- to 100-mm membrane. Following the removal of the waste product, the mixture solution was rinsed with PBS three times. Thus, ADSCs were collected and then added with DMEM containing 1% antibiotics-antimicotics and 10% fetal bovine serum (FBS) (Gibco-BRL). To resolve the erythrocytes, the cell suspension was mixed with 4% acetic acid (Merck, NJ, USA) at a ratio of 1:1.
The number of ADSCs with a nucleus was counted using hemocytometry, and they were aliquoted to culture vessels at a density of 1├Ś104/cm2. The cells were cultured at 37┬░C and 5% CO2, for which the culture medium was changed at a 2- to 3-day interval. At seven to ten days after the initial culture, we subcultured the cells when observing approximately 70-80% of them grew in culture vessels, for which we isolated them using 0.25% trypsin/EDTA (Gibco-BRL) and seeded them on the culture plate at a density of 1├Ś104/cm2. After subculturing the cells 2 to 3 times, we used them for the laboratory procedure.
Superparamagnetic iron oxide (SPIO) is used to label cells because it can be traced for long periods of time. Of various types of SPIO agents, ferumoxide (Feridex®) (Taejoon Pharm, Korea) which has become first commercially available for laboatory use; it was used in the current experiment.
The subcultured ADSCs were placed in culture vessels at a concentration of 10 ng/mL and then cultured for six hours, ensuring that they should ingest iron particles. They were isolated using 0.25% trypsin/EDTA and 2├Ś106 of them were suspended in PBS 0.3 mL. Thus, we prepared the ADSCs for the current laboratory procedure.
To examine the in vitro effects of ADSCs in improving cisplatin-induced nephrotoxicity, we treated mouse renal tubular epithelial cells (TCMK-1) (American Type Culture Collection) with both ADSCs and cisplatin mouse renal tubular epithelial cells.
To isolate the RNA for the real time reverse transcriptase-polymerase chain reaction (RT-PCR), TCMK-1 cell lines were placed in a 6-well plate at a concentration of 1├Ś106 cells per well and then cultured for two days. In the same method as above, we treated them with both cisplatin and ADSCs. Twenty-four hours later, the insert and culture medium were removed. To extract RNA, TCMK-1 cell lines were assayed using the easy-BLUETM Total RNA Extraction Kit (iNtRON Biotechnology Inc., Kyounggi, Korea) at a concentration of 800 mL per well. The real time RT-PCR was performed using Maxime RT-PCR PreMix tubes (iNtRON Biotechnology Inc.), for which the total concentration of RNA should be set at 1 mg in each tube containing a distilled water and total RNA sample. After the synthesis of cDNA in each tube, the following primers were added at a volume of 2 mL (10 pmol/mL) (total volume: 24 mL) as shown below:
Primers for TNF-╬▒:
5ŌĆÖ-GTC TAC TTT GGA GTC ATT GC-3ŌĆÖ (forward)
5ŌĆÖ-GAC ATT CGA GGC TCC AGT G-3ŌĆÖ (reverse)
Primers for Bcl-2:
5ŌĆÖ-ATC TTC TCC TTC CAG CCT GA-3ŌĆÖ (forward)
5ŌĆÖ-TCA GTC ATC CAC AGG GCG AT-3ŌĆÖ (reverse)
After the PCR (Hybaid, CA, USA), the sample was loaded on 1.5% agarose gel electrophoresis using RedsafeŌäó Nucleic acid staining solution (iNtRON Biotechnology Inc.). Thus, the electrophoresis was performed for 30 minutes at a voltage of 100 V. This was followed by assay of gel slices.
We divided 30 male white Sprague-Dawley (SD) rats (Central Lab. Animal Inc., Korea), weighing 270┬▒30 g and aged 12 weeks, into the following three groups:
(1) The control group (n=10): The rats receiving an intraperitoneal injection of a sterile saline.
(2) The cisplatin group (n=10): The rats receiving an intraperitoneal injection of cisplatin solution 3 mg/kg.
(3) The ADSC group (n=10): The rats receiving an intraperitoneal injection of cisplatin solution 3 mg/kgand one dose of intravenous injection of hADSC at a concentration of 2├Ś106/0.3mL via the caudal vein.
Cisplatin was diluted in a saline and then prepared as a solution with a concentration of 0.25 mg/mL. It was administered using a 5-mL synringe with a 23-G needle. To prepare the cisplatin solution, cisplatin and a saline were mixed at a ratio of 1:1 to a total concentration of 0.25 mg/mL. One day before the injection of ADSCs, we induced the occurrence of AKI using cisplatin. Twenty-four hours later, the rats of the ADSC group were given the cells at a concentration of 2├Ś106/0.3 mL via the caudal vein. For comparison, a saline was administered via the caudal vein to the rats of the cisplatin group and the control group at the same volume.
At five days after the treatment with cisplatin, a 3-mL blood sampling was done from the abdominal artery of the experimental rats of each group. The serum levels of BUN and creatinine were measured using AU 5400 Chemistry Analyzer (Olympus Optical co Ltd., Tokyo, Japan) and then averaged for comparison.
To find out whether there is a statistically meaningful enforcement of the Kolmogorov-Smirnov Normality test based on the results of preliminary experiments were conducted at 10 confirmed that a statistical analysis is possible for each group.
Following the sacrifice of the rats, the kidney of the rats was extracted and then examined using both light and electron microscopy. For light microscopy, paraffinembedded blocks were prepared, for which the kidney was fixed in 10% neutral formalin and then dehydrated using a series of ethanol. Following this, 4-mm sections were attached to the glass slide. This was followed by the deparaffinization and hydration. Then, we performed hematoxylineosin (H-E) staining and Prussian blue staining to detect iron particles. This was followed by the examination using the LM 6000D (Leica, Wetzlar, Germany). For Prussian-blue staining, the sample was reacted with a 1:1 mixture of 1% and 2% potassium ferrocyanide for ten minutes and then rinsed with water. For background staining, the sample was reacted with nuclear fast red for a minute.
On each slide, to examine the degree of damage to the kidney, we measured the width of the areas where there was a concurrent presence of the renal tubular necrosis. Five regions of the renal tissue were randomly selected. We drew a line vertically to the external membrane of the kidney and then measured the length extending from the junction between the cortex and medulla of the kidney to the external membrane of the kidney. We also measured the length of the area where there was a concurrent presence of the necrosis, thus comparing the ratio among the three groups. Thus, we obtained the ratio of the renal injury to the overall cortex. This measurement method was designed by ourselves, because we realized that the injury area of this experiment was started from and limited to corticomedullary junction through the microscopic examination. So we thought to apply individual injured epithelial cell counts method randomly, which have been used generally, was not enough to demonstrate this injury pattern.
For electron microscopy, we performed pre-fixation using 2.5% glutaraldehyde (Polyscience, Europe GmbH, Eppelheim, Germany) for three hours and then did post-fixation using 1% osmmium teraoxide (Polyscience Europe GmbH) for an hour and 30 minutes. We performed subsequent dehydration using ethyl alcohol (Merck KGaA, Darmstadt, Germany). This was followed by the infiltration of propylene oxide (Merck). The sample was embedded in epon resin (Polyscience Europe GmbH) and then subjected to a 48-hr polymerization in a 60┬░C incubator. Thus, the block was prepared. The sample was sectioned using the Ultra microtome (Leica) at a thickness of 80 nm and then double stained using uranyl acetate (Polyscience Europe GmbH) and lead nitrate (Polyscience Europe GmbH). This was followed by the examination using transmission electron microscopy with H-7000 (Hitachi, Tokyo, Japan) at a voltage of 75 kV.
Each group comprises ten rats, for which the serum levels of BUN and creatinine were compared using student t-test. Statistical analysis was done using the SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). We also compared the extent of the renal damage on light microscopy between the two groups. A P-value of <0.05 was considered statistically significant.
The current study was approved by the Institutional Review Board (IRB) of Yeungnam University Medical Center for the culture and proliferation of ADSCs (IRB approval protocol No. PCR-09-82) and by the Institutional Animal Care and Use Committee of Yeungnam University School of Medicine (YUMC AEC 2010-005) for animal experiments. We obtained a written informed consent from patients for the use of hADSC in the current experiment.
The TNF-╬▒ and Bcl-2 mRNA expressions were examined by polymerase chain reaction (PCR) (Fig. 1A). The degree of the expression of TNF-╬▒ of the cisplatin group was significantly higher than the ADSC group (283% vs. 123%, P<0.05). Moreover, the degree of the expression of Bcl-2 was significantly lower in the cisplatin group and as compared with the ADSC group (19% vs. 42%, P<0.05) (Fig. 1B).
The serum levels of BUN were 16.3┬▒1.4 mg/dL in the control group, 94.4┬▒28.9 mg/dL in the cisplatin group and 61.2┬▒32.4 mg/dL in the ADSC group (Fig. 2A). In addition, the serum levels of creatinine were 0.51┬▒0.08 mg/dL, 2.66┬▒0.71 mg/dL and 1.67┬▒0.99 mg/dL (Fig. 2B). This showed that serum levels of BUN and creatinine were significantly higher in the cisplatin group as compared with the ADSC group (P=0.027 and P=0.02, respectively) (Fig. 2).
On light microscopy, the necrotic renal injury was commonly characterized by the detachment of tubular epithelial cells from the basement membrane. The detached cells formed amorphous clump due to necrosis and filled the lumen of the renal tubules. The regenerated cells were lower and slightly wider than normal cells. The degree of necrosis was the highest at the junction between the cortex and medulla. The necrosis was mainly seen in the proximal renal tubule. We also measured the length of the area where there was a concurrent presence of the necrosis (Fig. 3A), thus comparing the ratio among the three groups. (Fig. 3B; control group, Fig. 3C; cisplatin group, Fig. 3D; ADSC group). In the current experiment, there were almost no damages to the superior region of the renal cortex.
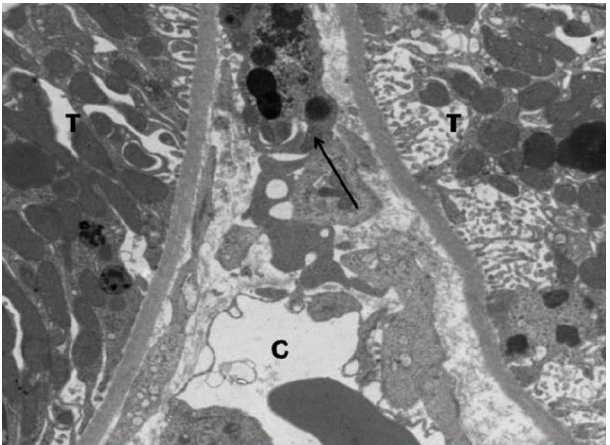
On electron microscopy, necrotic epithelial cells had a nuclear destruction and damages to cell organelles. Regenerated cells had a smaller amount of cell organelles and smaller nuclei, but they had a similar shape to the normal cells. There were capillaries extended from the interstitium between the epithelial cells. In the adjacent areas, cell-like structures containing SPIO-labeled iron particles are intermittently present. There were capillaries (C) extended from the interstitium between the epithelial cells. (Arrow) In the adjacent areas, cell-like structures containing SPIO-labeled iron particles are intermittently present between the tubular epithelial cells (T) (┬┤7,000) (Fig. 4).
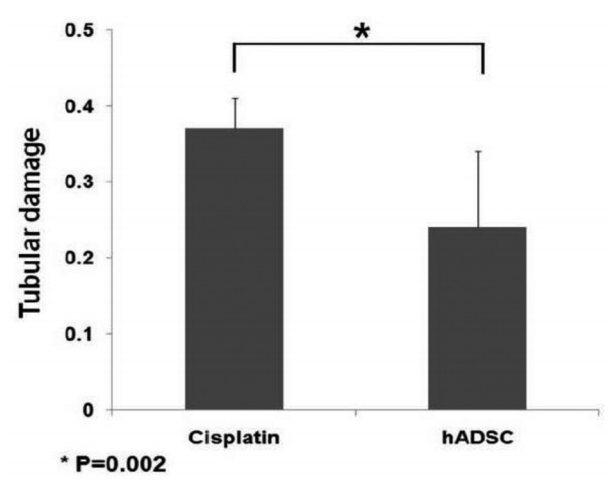
In the control group, there were no findings that are suggestive of necrosis. But the ratio of the renal injury to the overall cortex was 0.37┬▒0.04 in the cisplatin group and 0.24┬▒0.1 in the ADSC group (Fig. 5). This difference reached statistical significance (P=0.002).
The pathophysiology of AKIis presumed to be the detachment of the epithelial cells from the basement membrane of the renal tubules [12]. The regeneration of the detached tubular epithelial cells begins at two days after the onset of the injury. Ten days later, approximately 50% of the detached epithelial cells are regenerated [17]. It takes approximately four weeks to achieve a complete recovery of the morphological characteristics [18,19]. There are no significant decreases in the overall mortality, for which ongoing studies are conducted [7]. In recent years, stem cell studies have been actively conducted; several authors have examined whether stem cells would be involved in the regeneration of tubular epithelial cells in patients with AKI [11,12].
In an animal model of cisplatin-induced AKI, its dose has been reported to vary depending on the authors. Nishida et al. performed a subcutaneous injection of cisplatin 15 mg/kg in rats [2]. In addition, Morigi et al. also performed a subcutaneous injection of cisplatin 12.7 mg/kg and thereby induced the occurrence of acute renal injury [7]. Greene et al. injected varying doses of cisplatin, ranging from 2.5 to 7.5 mg/kg, and thereby induced the occurrence of acute renal injury [20]. Suddek performed an intraabdominal injection of cisplatin 6 mg/kg and thereby induced the occurrence of acute renal injury [21]. But we performed preliminary experiments to adjust the amount of dosing, and therefore found that there were significant changes in serum biochemical and histopathologic findings of the kidney following a high-dose (5 mg/kg) of cisplatin treatment. In addition, we also found that there were findings that are suggestive of chemical peritonitis following a high-dose of cisplatin treatment. We therefore performed an intraabdominal injection of cisplatin 3 mg/kg (diluted to 0.25 mg/ml). Thus, we were successful in creating an animal experimental model of cisplatin-induced AKI.
In the current experiment, the degree of the expression of TNF-╬▒ was the highest in the cisplatin group. In addition, it was higher by 123% in the ADSC group as compared with the control group. Moreover, the degree of the expression of Bcl-2 was higher in the ADSC group (42%) as compared with the cisplatin group (19%). These findings suggest that the stem cells have anti-inflammatory and anti-apoptotic effects.
In the experimental group, on light microscopy, there were necrosis and detachment of the epithelial cells lining the renal tubules. Of note, the degree of the necrosis was the highest at the junction between the cortex and medulla. To objectively evaluate these findings, we measured the width of the necrotic renal injury, thus calculating the ratio of the renal injury to the overall cortex. In the ADSC group, 35% of the cells achieved a recovery. In addition, the serum levels of BUN and creatinine were significantly lower. These findings suggest that the stem cells might have an effect in improving damages to the acute renal tubules.
To date, several authors have reported that the stem cells have an effect in improving the symptoms of acute renal failure. In summary, there are major reports in this series; (1) the stem cells had a direct effect in regenerating the damaged epithelial cells, (2) they had a local effect in regenerating the damaged cells around them and (3) they had their own systemic effects. Lin et al. maintained that the stem cells had a direct effect in regenerating the damaged epithelial cells [11]. Morigi et al. reported that there were Y-chromosomes following the administration of mesenchymal stem cells harvested from male white rats in an animal model of cisplatin-induced renal injury using female white rats, thus maintaining that the stem cells had a direct effect in regenerating the damaged [7]. But this has not been accepted because these authors identified an extremely small number of the regenerated cells and the experimental procedure has not been reproduced by other authors. With regard to the systemic effects of the stem cells, it has been maintained that a massive amount the stem cells secrete substances and thereby have an effect in regenerating the damaged kidney or they are involved in the indirect regeneration of the damaged kidney by stimulating other organs than the kidney. This has been of increasing interest as recent studies have revealed the presence of various types of cytokines or Ki-67-positive cells [23]. In addition, Caplan and Dennis reported that the stem cells secreted bioactive factors that are indirectly involved in the recovery and regeneration of the damaged sites on histopathologic examinations of ischemic injury.
Limitations of this study were to write down the numbers included in the experiment, in the future by using more objects are thought to derive think you better results. Besides, by varying the amount of cisplatin, by varying the degree of damage of them, it would be nice if you compare the effect of the stem cell. In addition, using other drugs in addition to cisplatin is considered to be helpful to compare the effect of stem cell is also an experiment.
To summarize, our results are as follows:
(1) The degree of the expression of TNF-╬▒ and that of Bcl-2 were significantly higher and lower, respectively, in the cisplatin group as compared with the ADSC group (P<0.05).
(2) Serum levels of BUN and creatinine were significantly higher in the cisplatin group as compared with the ADSC group (P=0.027 and P=0.02, respectively).
(3) On histopathologic examinations, there was a significant difference in the ratio of the renal injury between the cisplatin group and the ADSC group (P=0.002).
Based on the above results, it can be concluded that the ADSCs might have a beneficial effect in regenerating the damaged renal tubular epithelial cells.
Acknowledgement
This work was supported by the LG Life Science and by Chunma Medical Research Foundation (2010-1).
References
1. Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004;114:5-14.



2. Nishida M, Fujimoto S, Toiyama K, Sato H, Hamaoka K. Effect of hematopoietic cytokines on renal function in cisplatin-induced ARF in mice. Biochem Biophys Res Commun 2004;324:341-7.

3. Joannidis M, Forni LG. Clinical review: timing of renal replacement therapy. Crit Care 2011;15:223.



4. Garcovich M1, Zocco MA, Gasbarrini A. Clinical use of albumin in hepatology. Blood Transfus 2009;7:268-77.


5. Cadwallader AB1, de la Torre X, Tieri A, Botr├© F. The abuse of diuretics as performance-enhancing drugs and masking agents in sport doping: pharmacology, toxicology and analysis. Br J Pharmacol 2010;161:1-16.



7. Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 2004;15:1794-804.


8. Cimino GD, Pan CX, Henderson PT. Personalized medicine for targeted and platinum-based chemotherapy of lung and bladder cancer. Bioanalysis 2013;5:369-91.



9. Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 2012;64:706-21.



10. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838-43.


11. Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 2003;14:1188-99.


12. Chhabra P, Brayman KL. The use of stem cells in kidney disease. Curr Opin Organ Transplant 2009;14:72-8.


13. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 2003;5:362-9.


14. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279-95.



15. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 2008;45:115-20.



16. Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007;18:2486-96.


17. Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, et al. Identification and kinetics of leukocytes after severe ischemia/reperfusion renal injury. Nephrol Dial Transplant 2000;15:1562-74.


18. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 2001;281:F887-F899.

20. Greene SN, Ramos-Vara JA, Craig BA, Hooser SB, Anderson C, Fourez LM, et al. Effects of cyclooxygenase inhibitor treatment on the renal toxicity of cisplatin in rats. Cancer Chemother Pharmacol 2010;65:549-56.


21. Suddek GM. Sunitinib improves chemotherapeutic efficacy and ameliorates cisplatin-induced nephrotoxicity in experimental animals. Cancer Chemother Pharmacol 2011;67:1035-44.


22. Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med 2008;59:311-25.


Fig.┬Ā1.
TNF-╬▒ and Bcl-2 mRNA expressions. (A) Representative agarose gel electrophoretic patterns of PCR products were shown. (B) The degree of the expression of TNF-╬▒ was significantly higher by 283% in the cisplatin group and 123% in the ADSC group as compared with the control group (P<0.05). Moreover, the degree of the expression of Bcl-2 was significantly lower by 19% in the cisplatin group and 42% in the ADSC group as compared with the control group (P<0.05).
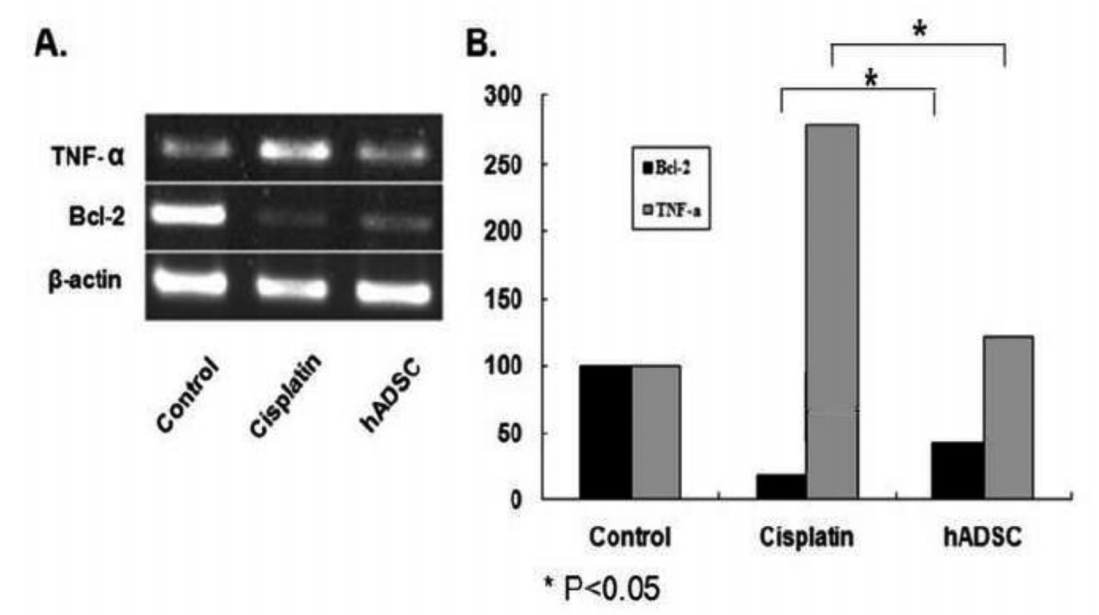
Fig.┬Ā2.
The effects of the adipose-derived stem cells on the renal functions. (A) The serum levels of BUN were 16.3┬▒1.4 mg/dL in the control group, 94.4┬▒28.9 mg/dL in the cisplatin group and 61.2┬▒32.4 mg/dL in the ADSC group. (B) The serum levels of creatinine were 0.51┬▒0.08 mg/dL, 2.66┬▒0.71 mg/dL and 1.67┬▒0.99 mg/dL in the corresponding order. This showed that serum levels of BUN and creatinine were significantly higher in the ADSC group as compared with the control group (P=0.027 and P=0.02, respectively). Error bars represent mean┬▒standard deviations.
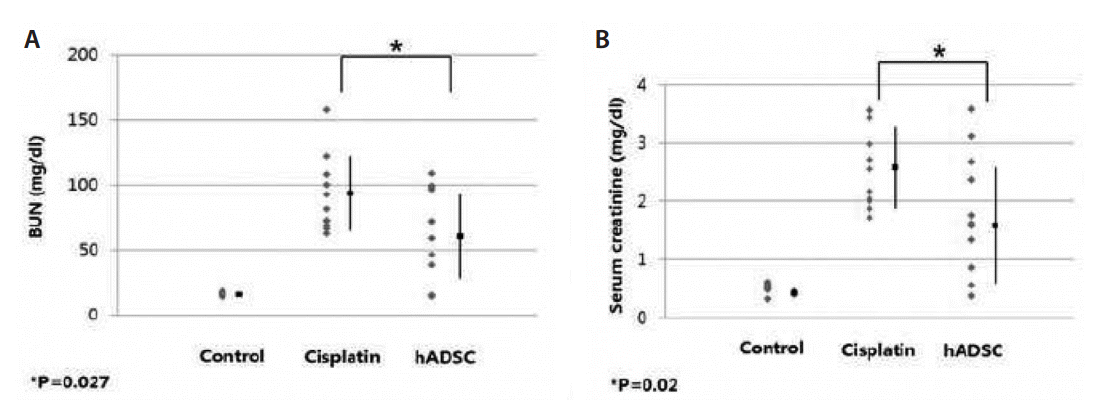
Fig.┬Ā3.
Histopathologic findings. (A) Five regions of the renal tissue were randomly selected. We drew a line vertically to the external membrane of the kidney and then measured the length extending from the junction between the cortex and medulla of the kidney to the external membrane of the kidney. We also measured the length of the area where there was a concurrent presence of the necrosis, thus comparing the ratio among the three groups. Thus, we obtained the ratio of the renal injury (b) to the overall cortex (a). (B) The control group. (C) The cisplatin group. (D) The ADSC group.

Fig.┬Ā4.
Electron microscopy findings. Necrotic epithelial cells had a nuclear destruction and damages to cell organelles. Regenerated cells had a smaller amount of cell organelles and smaller nuclei, but they had a similar shape to the normal cells. There were capillaries (C) extended from the interstitium between the epithelial cells. (Arrow) In the adjacent areas, cell-like structures containing SPIO-labeled iron particles are intermittently present between the tubular epithelial cells (T) (┬┤7,000).
- TOOLS
-
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link XML Download
XML Download Full text via DOI
Full text via DOI-
 Download Citation
Download Citation
- Download Citation
-
- Close
 Print
Print-
Share :