Introduction
Proteinuria has long been one of the most important targets of therapy for many kidney diseases. However, only a small number of studies have been performed that analyzed urinary proteins in comparison with serum proteins in glomerular diseases.
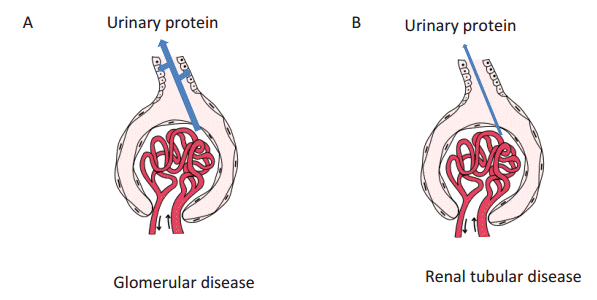
Urinary proteins in glomerular diseases are those proteins that pass through the glomerular capillary walls in considerable amounts, and are then eliminated via reabsorption by the renal proximal tubules. Fig. 1A (arrows) shows the path of proteins in primary urine; the proteins pass through the glomerular capillary walls, then attach, and are reabsorbed by the renal proximal tubules. The proteins eliminated by reabsorption are then concentrated and voided as final urinary proteins. On the other hand, in renal tubular diseases, the urinary proteins consist of proteins that pass through the glomerular capillary walls in very small amounts, but are not reabsorbed by the renal proximal tubules, and are concentrated and voided into the final urine (Fig. 1B). It is necessary to understand these processes better in order to elucidate the charge-selective protein sieving function of the glomerular capillary walls.
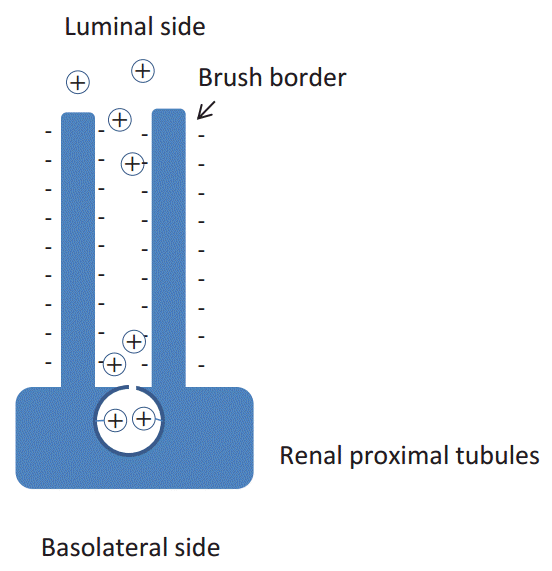
Cationic charge-preferential protein reabsorption in renal proximal tubules
The megalinŌĆÉcubilin complex has been found to play a central role in protein reabsorption in the proximal tubules, and serves as a multi-ligand receptor [1]. However, another receptor located in the proximal tubules also binds to immunoglobulin G (IgG) (Fig. 2) [2]. This receptor is identical to the neonatal Fc╬│ receptor (FcRN). FcRN is expressed in the human placenta, and is known to transport IgG from mother to the fetus by means of transcytosis. Recently, several papers have postulated that this FcRN also transports albumin in the proximal tubules for recycling of albumin [3]. However, it has long been hypothesized that a cationic charge-preferential reabsorption mechanism is present in the renal proximal tubules (Fig. 3) [4] because the microvilli of the renal proximal tubular cells are negatively charged and some parts of the filtered proteins are thought to be positively charged because of the acidification of urine in the proximal tubules. To provide evidence in support of this hypothesis, we sought to evaluate the charge diversity of serum and urinary IgG.
Discussion
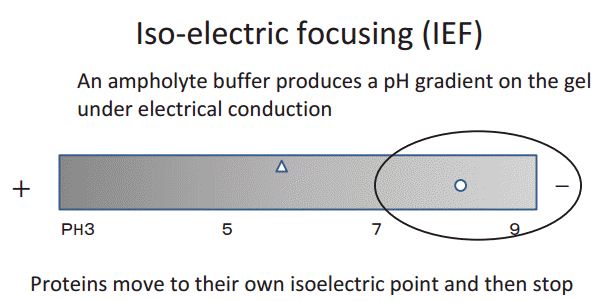
Prior to discussing the results of the present study, it is necessary to understand the principle of isoelectric focusing, as shown in Fig. 4. The isoelectric point (pI) is the pH at which a protein does not carry a net electrical charge. Proteins are composed of amino acids and sugars, which are basic, neutral, or acidic in nature. The pH of the urine falls to 6 in the renal proximal tubules. At this pH, the cationic portion of IgG (pI 8ŌĆō9) is strongly positively charged, and seems to attach more easily to the negatively charged microvilli of the renal proximal tubules.
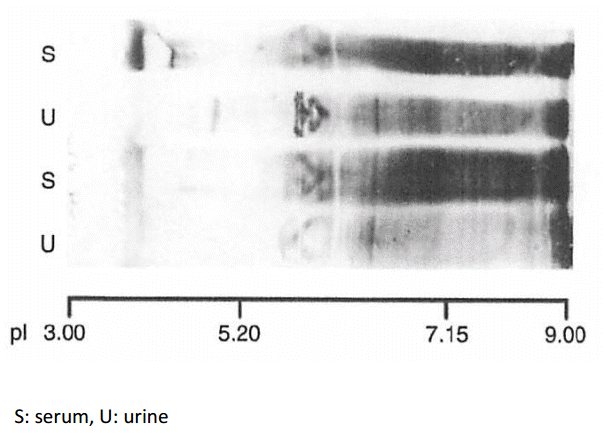
In order to show cationic chargeŌĆÉpreferential protein reabsorption in the renal tubules, we utilized the characteristics of the very broad charge diversity of IgG. As shown in Fig. 5 (square box), the cationic portion of IgG (pI 8ŌĆō9; indicated by arrow) is present in the serum (S) but absent in the urine (U).
IgG has been shown to be reabsorbed in the renal proximal tubules by FcRN in vitro. This process is inhibited if the pH of the culture medium is raised (as the cationic charge decreases) [2].
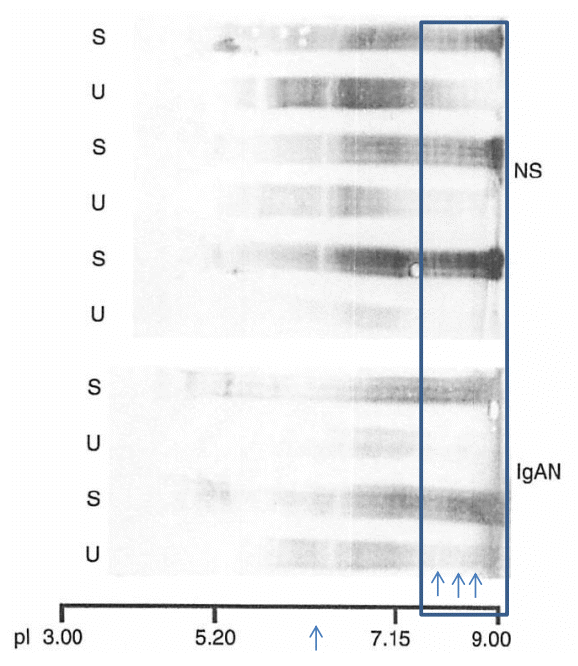
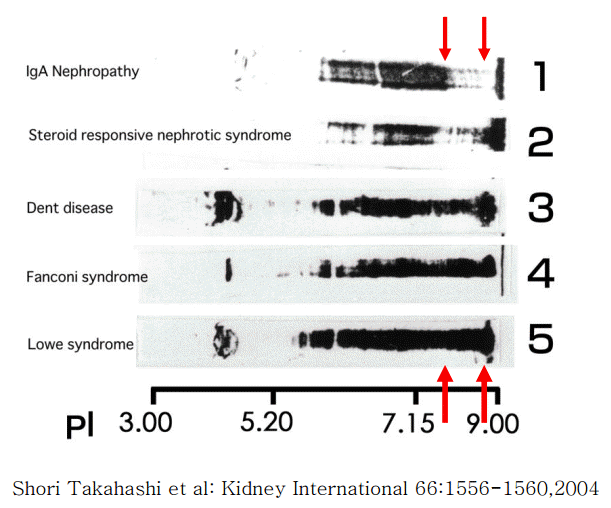
In cases of Dent disease showing proximal tubular proteinuria, the charge diversities of serum and urinary IgGs have been observed to be almost identical (Fig. 6). This indicates that the cationic charge-preferential protein reabsorption mechanism is not functional in Dent disease. This difference in the charge diversities of urinary IgG in glomerular diseases compared to those in renal tubular diseases is apparent. The cationic portion of IgG that is absent in glomerular diseases (upper arrow), but is present in renal tubular diseases (lower arrow). This suggests that the cationic portion of IgG is reabsorbed in the renal proximal tubules only with the renal proximal tubular function intact (Fig. 7) [5].
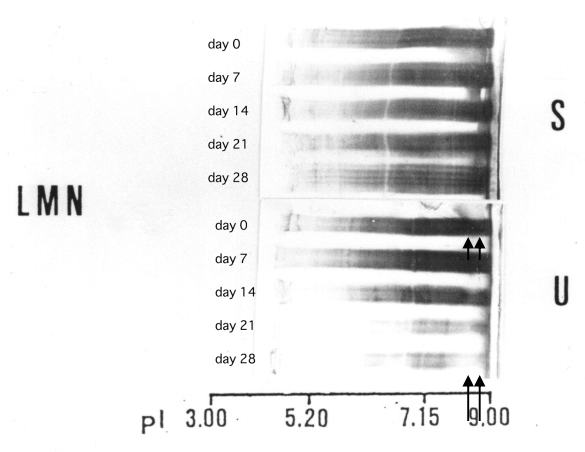
In a patient with lupus membranous nephropathy (LMN) with extremely severe proteinuria, the charge diversity between serum and urinary IgG did not show any difference (Fig. 8, upper arrows). However, during remission, the cationic part of IgG in the urine became predominantly faint (Fig. 8, lower arrows). This indicates that the reabsorption of IgG may be a saturable process [5].
Charge selectivity index showed differences in the glomerular charge sieving function between healthy subjects, patients with podocyte disease, and patients with glomerulonephritis
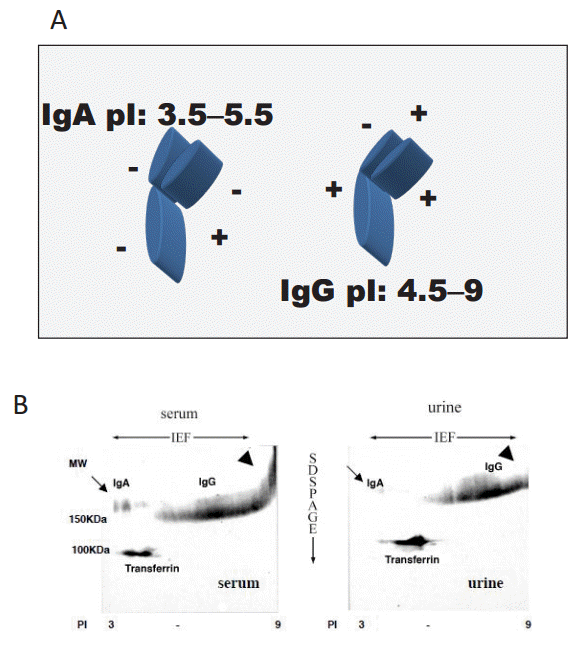
Next, we investigated the charge selectivity of the glomerular capillary walls by analyzing serum and urinary IgA and IgG. IgA has a StokesŌĆÉEinstein (S-E) radius of 61 ├ģ and low pI (3.5ŌĆō5.5), while IgG has a StokesŌĆÉEinstein radius of 49ŌĆō60 ├ģ and high pI (4.5ŌĆō9) (Fig. 9A). Therefore, the molecules have similar tertiary structure and S-E radius, but distinct isoelectric points. Fig. 9B shows 2-dimensional (2D) electrophoresis [6] isoelectric focusing (IEF) for the horizontal dimension, and sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis for the vertical dimension), followed by immunoblotting of IgA and IgG (2D immunoblotting of IgA and IgG). This figure clearly illustrates the distinct difference in molecular charge between IgA and IgG.
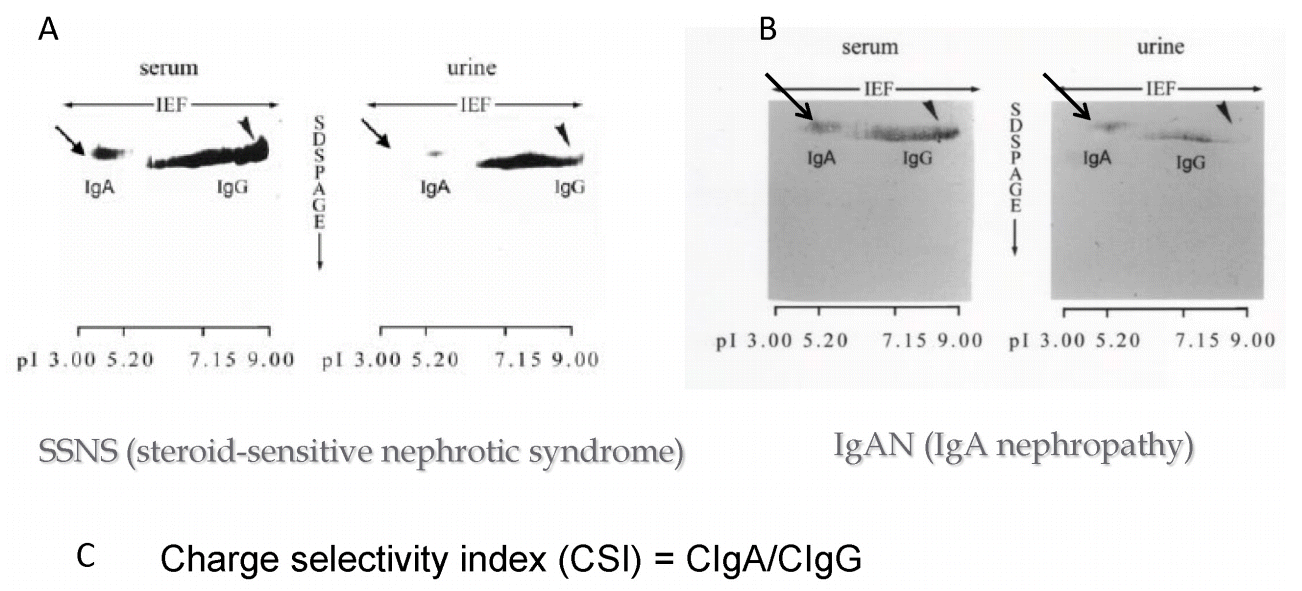
Fig. 10 shows the 2D immunoblotting of serum and urinary IgA and IgG in patients with steroidŌĆÉsensitive nephrotic syndrome (SSNS; Fig. 10A) and IgA nephropathy (IgAN; Fig. 10B). It is obvious that the density of IgA in the urine of patient with SSNS is much weaker than that of IgAN in comparison with that of urinary IgG in both diseases. These results indicate that the fractional clearance of IgA to IgG could serve as a charge selectivity index (CSI) of the glomerular capillary walls (Fig. 10C) [7].
However, it was difficult to determine how to evaluate the CSI of healthy subjects. Thus, we chose to use the CSI of patients with Dent disease instead of healthy subjects, since patients with Dent disease are considered to have a normal glomerular sieving function, but defective proximal tubular protein reabsorption [8]. Therefore, the final urine of patients with Dent disease could be considered a concentrate of the primary urine eliminating the influence of reabsorption through the renal proximal tubules.
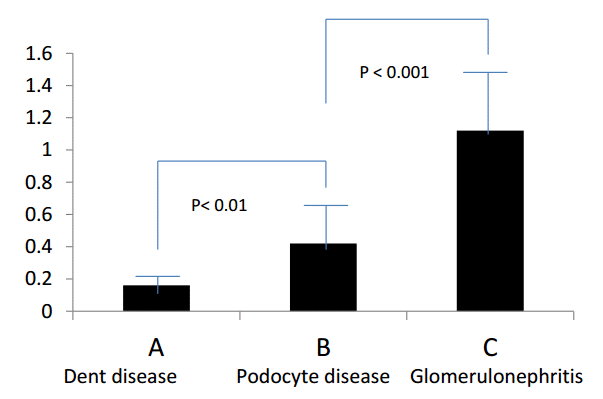
We investigated the differences in charge selectivity in the glomerular capillary walls using CSI (Fig. 10C). The CSI was investigated in eight patients with Dent disease (Group A), in 40 patients with podocyte disease (Group B: SSNS 35 patients, focal segmental glomerulosclerosis 4 patients, and Finnish type congenital nephrotic syndrome 1 patient), and in 75 patients with chronic glomerulonephritis (Group C: IgAN 41 patients, purpura nephritis 21 patients, membranoproliferative glomerulonephritis 5 patients, and Alport syndrome 8 patients). The results showed a statistically significant difference in CSI between each group (Table 1 and Fig. 11) [7].
Conclusion
The cationic portion of IgG is reabsorbed in the normal renal proximal tubules, in a cationic chargeŌĆÉpreferential manner, and the reabsorption of cationic IgG seems to be a saturable process. The chargeŌĆÉselective protein sieving function is operational in healthy subjects, impaired in podocyte disease, and is lacking in patients affected by glomerulonephritis.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print